Efficacy and Safety of Switching Patients Inadequately Controlled on Basal Insulin to Insulin Glargine 300 U/mL: The TRANSITION 2 Study
- PMID: 31782050
- PMCID: PMC6965550
- DOI: 10.1007/s13300-019-00734-8
Efficacy and Safety of Switching Patients Inadequately Controlled on Basal Insulin to Insulin Glargine 300 U/mL: The TRANSITION 2 Study
Abstract
Introduction: This study aimed to determine, in close to real-life conditions, the efficacy and safety of switching from any basal insulin to insulin glargine 300 U/mL (Gla-300) in patients with uncontrolled type 2 diabetes (T2D).
Methods: This was an interventional, multicenter, single-arm, prospective study with a 24-week treatment phase. Adult patients with T2D treated with basal insulin with or without other antidiabetics, HbA1c > 7.5%, and fasting self-monitored blood glucose (F-SMBG) > 130 mg/dL (mean of three measures) at baseline were included. Insulin dose was titrated to reach F-SMBG 90-130 mg/dL. Efficacy and safety were assessed at 12 weeks (W12) and 24 weeks (W24). The main outcome parameter was HbA1c change between baseline and W24. Safety parameters included self-reported hypoglycemia (any type). Patients' satisfaction with the treatment was assessed by the Diabetes Treatment Satisfaction Questionnaire (DTSQ).
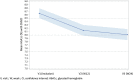
Results: A total of 140 patients were included and 137 were treated. Mean HbA1c decreased from 8.64% at baseline to 8.14% at W12 (mean difference [95% CI] - 0.51% [- 0.64; - 0.38]) and 8.01% at W24 (- 0.64% [- 0.81; - 0.46]). _target F-SMBG was reached in 35.0% of the patients at W12 and 38.4% at W24. The percentages of patients reaching HbA1c levels < 7.0%, < 7.5%, and < 8.0% at W24 were 11.4%, 29.5%, and 50.8%, respectively, while only 31.6% had an HbA1c value < 8.0% at baseline. HbA1c reduction was greater in patients with higher baseline levels. During the treatment phase, 46.0% of the participants had at least one hypoglycemia event; 31.4% documented symptomatic hypoglycemia, 2.2% severe hypoglycemia, and 12.2% nocturnal hypoglycemia. Treatment satisfaction increased by 20% between baseline and W24.
Conclusion: These data, derived from close to real-life practice in France, confirm the reassuring results of randomized trials on the efficacy and safety of Gla-300.
Trial registration: EudraCT number 2015-002416-33.
Keywords: Gla-300; Glargine; Hypoglycemia; Insulin; Real-life; Real-world evidence; Type 2 diabetes.
Figures
Similar articles
-
Impact of Switching from Twice-Daily Basal Insulin to Once-Daily Insulin Glargine 300 U/mL in People with Type 1 Diabetes on Basal-Bolus Insulin: Phase 4 OPTIMIZE Study.Diabetes Ther. 2020 Feb;11(2):495-507. doi: 10.1007/s13300-019-00749-1. Epub 2020 Jan 10. Diabetes Ther. 2020. PMID: 31925722 Free PMC article.
-
Effect of switching from twice-daily basal insulin to once-daily insulin glargine 300 U/mL (Gla-300) in Brazilian people with type 1 diabetes.Diabetol Metab Syndr. 2024 Jul 9;16(1):152. doi: 10.1186/s13098-024-01385-x. Diabetol Metab Syndr. 2024. PMID: 38982528 Free PMC article.
-
Improved Glycemic Control with Insulin Glargine 300 U/mL (Toujeo®) in Patients with Type 2 Diabetes: Real-World Effectiveness in Switzerland.Diabetes Ther. 2018 Dec;9(6):2325-2334. doi: 10.1007/s13300-018-0518-x. Epub 2018 Oct 9. Diabetes Ther. 2018. PMID: 30302721 Free PMC article.
-
Efficacy and safety of insulin glargine 300 U/mL (Gla-300) during hospitalization and therapy intensification at discharge in patients with insufficiently controlled type 2 diabetes: results of the phase IV COBALTA trial.BMJ Open Diabetes Res Care. 2020 Sep;8(1):e001518. doi: 10.1136/bmjdrc-2020-001518. BMJ Open Diabetes Res Care. 2020. PMID: 32928792 Free PMC article. Clinical Trial.
-
Insulin glargine: a systematic review of a long-acting insulin analogue.Clin Ther. 2003 Jun;25(6):1541-77, discussion 1539-40. doi: 10.1016/s0149-2918(03)80156-x. Clin Ther. 2003. PMID: 12860485 Review.
Cited by
-
Impact of Age on the Effectiveness and Safety of Insulin Glargine 300 U/mL: Results from the REALI European Pooled Data Analysis.Diabetes Ther. 2021 Apr;12(4):1073-1097. doi: 10.1007/s13300-021-01030-0. Epub 2021 Mar 1. Diabetes Ther. 2021. PMID: 33650085 Free PMC article.
-
Glycaemic Control in People with Type 2 Diabetes Mellitus Switching from Basal Insulin to Insulin Glargine 300 U/ml (Gla-300): Results from the REALI Pooled Database.Diabetes Ther. 2023 Feb;14(2):401-413. doi: 10.1007/s13300-022-01356-3. Epub 2023 Jan 4. Diabetes Ther. 2023. PMID: 36596946 Free PMC article.
-
Efficacy and Safety of Insulin Glargine 300 U/mL in People with Type 2 Diabetes Uncontrolled on Basal Insulin: The 26-Week Interventional, Single-Arm ARTEMIS-DM Study.Diabetes Ther. 2022 Jul;13(7):1395-1408. doi: 10.1007/s13300-022-01271-7. Epub 2022 Jun 17. Diabetes Ther. 2022. PMID: 35713873 Free PMC article.
-
Does Gender Influence the Effectiveness and Safety of Insulin Glargine 300 U/ml in Patients with Uncontrolled Type 2 Diabetes? Results from the REALI European Pooled Analysis.Diabetes Ther. 2022 Jan;13(1):57-73. doi: 10.1007/s13300-021-01179-8. Epub 2021 Nov 16. Diabetes Ther. 2022. PMID: 34784005 Free PMC article.
-
Glycaemic Control with Insulin Glargine 300 U/mL in Individuals with Type 2 Diabetes and Chronic Kidney Disease: A REALI European Pooled Data Analysis.Diabetes Ther. 2021 Apr;12(4):1159-1174. doi: 10.1007/s13300-021-01031-z. Epub 2021 Mar 9. Diabetes Ther. 2021. PMID: 33751403 Free PMC article.
References
-
- International Diabetes Federation. France: Country report 2017, The IDF Diabetes Atlas, 8th ed. http://reports.instantatlas.com/report/view/846e76122b5f476fa6ef09471965.... Accessed 26 Sept 2019.
-
- Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7:e015949. doi: 10.1136/bmjopen-2017-015949. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources