Cocaine Dysregulates Dynorphin Modulation of Inhibitory Neurotransmission in the Ventral Pallidum in a Cell-Type-Specific Manner
- PMID: 31836660
- PMCID: PMC7002149
- DOI: 10.1523/JNEUROSCI.1262-19.2019
Cocaine Dysregulates Dynorphin Modulation of Inhibitory Neurotransmission in the Ventral Pallidum in a Cell-Type-Specific Manner
Abstract
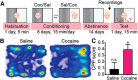
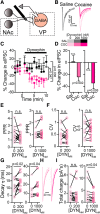
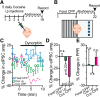
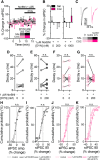
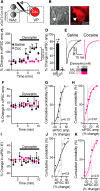
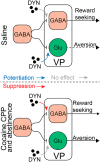
Cocaine-driven changes in the modulation of neurotransmission by neuromodulators are poorly understood. The ventral pallidum (VP) is a key structure in the reward system, in which GABA neurotransmission is regulated by opioid neuropeptides, including dynorphin. However, it is not known whether dynorphin acts differently on different cell types in the VP and whether its effects are altered by withdrawal from cocaine. Here, we trained wild-type, D1-Cre, A2A-Cre, or vGluT2-Cre:Ai9 male and female mice in a cocaine conditioned place preference protocol followed by 2 weeks of abstinence, and then recorded GABAergic synaptic input evoked either electrically or optogenetically onto identified VP neurons before and after applying dynorphin. We found that after cocaine CPP and abstinence dynorphin attenuated inhibitory input to VPGABA neurons through a postsynaptic mechanism. This effect was absent in saline mice. Furthermore, this effect was seen specifically on the inputs from nucleus accumbens medium spiny neurons expressing either the D1 or the D2 dopamine receptor. Unlike its effect on VPGABA neurons, dynorphin surprisingly potentiated the inhibitory input on VPvGluT2 neurons, but this effect was abolished after cocaine CPP and abstinence. Thus, dynorphin has contrasting influences on GABA input to VPGABA and VPvGluT2 neurons and these influences are affected differentially by cocaine CPP and abstinence. Collectively, our data suggest a role for dynorphin in withdrawal through its actions in the VP. As VPGABA and VPvGluT2 neurons have contrasting effects on drug-seeking behavior, our data may indicate a complex role for dynorphin in withdrawal from cocaine.SIGNIFICANCE STATEMENT The ventral pallidum consists mainly of GABAergic reward-promoting neurons, but it also encloses a subgroup of aversion-promoting glutamatergic neurons. Dynorphin, an opioid neuropeptide abundant in the ventral pallidum, shows differential modulation of GABA input to GABAergic and glutamatergic pallidal neurons and may therefore affect both the rewarding and aversive aspects of withdrawal. Indeed, abstinence after repeated exposure to cocaine alters dynorphin actions in a cell-type-specific manner; after abstinence dynorphin suppresses the inhibitory drive on the "rewarding" GABAergic neurons but ceases to modulate the inhibitory drive on the "aversive" glutamatergic neurons. This reflects a complex role for dynorphin in cocaine reward and abstinence.
Keywords: GABA; cocaine; dynorphin; electrophysiology; vGluT2 neurons; ventral pallidum.
Copyright © 2020 the authors.
Figures


Similar articles
-
Projection-Specific Potentiation of Ventral Pallidal Glutamatergic Outputs after Abstinence from Cocaine.J Neurosci. 2020 Feb 5;40(6):1276-1285. doi: 10.1523/JNEUROSCI.0929-19.2019. Epub 2019 Dec 13. J Neurosci. 2020. PMID: 31836662 Free PMC article.
-
Loss of Plasticity in the D2-Accumbens Pallidal Pathway Promotes Cocaine Seeking.J Neurosci. 2017 Jan 25;37(4):757-767. doi: 10.1523/JNEUROSCI.2659-16.2016. J Neurosci. 2017. PMID: 28123013 Free PMC article.
-
Cocaine induces input and cell-type-specific synaptic plasticity in ventral pallidum-projecting nucleus accumbens medium spiny neurons.Neuropsychopharmacology. 2022 Jul;47(8):1461-1472. doi: 10.1038/s41386-022-01285-6. Epub 2022 Feb 4. Neuropsychopharmacology. 2022. PMID: 35121830 Free PMC article.
-
Ventral pallidal modulation of aversion processing.Brain Res. 2019 Jun 15;1713:62-69. doi: 10.1016/j.brainres.2018.10.010. Epub 2018 Oct 6. Brain Res. 2019. PMID: 30300634 Review.
-
Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens.Mol Psychiatry. 2022 Jan;27(1):669-686. doi: 10.1038/s41380-021-01112-2. Epub 2021 May 7. Mol Psychiatry. 2022. PMID: 33963288 Free PMC article. Review.
Cited by
-
Traumatic Stress-Induced Vulnerability to Addiction: Critical Role of the Dynorphin/Kappa Opioid Receptor System.Front Pharmacol. 2022 Apr 27;13:856672. doi: 10.3389/fphar.2022.856672. eCollection 2022. Front Pharmacol. 2022. PMID: 35571111 Free PMC article. Review.
-
Ventral pallidum GABA neurons bidirectionally control opioid relapse across rat behavioral models.Addict Neurosci. 2022 Sep;3:100026. doi: 10.1016/j.addicn.2022.100026. Epub 2022 Jun 30. Addict Neurosci. 2022. PMID: 36156918 Free PMC article.
-
Opioid Receptor-Mediated Regulation of Neurotransmission in the Brain.Front Mol Neurosci. 2022 Jun 15;15:919773. doi: 10.3389/fnmol.2022.919773. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35782382 Free PMC article. Review.
-
Ventral pallidum GABA and glutamate neurons drive approach and avoidance through distinct modulation of VTA cell types.Nat Commun. 2024 May 18;15(1):4233. doi: 10.1038/s41467-024-48340-y. Nat Commun. 2024. PMID: 38762463 Free PMC article.
-
Metaplasticity in the Ventral Pallidum as a Potential Marker for the Propensity to Gain Weight in Chronic High-Calorie Diet.J Neurosci. 2020 Dec 9;40(50):9725-9735. doi: 10.1523/JNEUROSCI.1809-20.2020. Epub 2020 Nov 16. J Neurosci. 2020. PMID: 33199503 Free PMC article.
References
-
- Allen Institute for Brain Science (2004) Allen mouse brain atlas. Retrieved November 27, 2018. Available at http://mouse.brain-map.org/gene/show/18156.
-
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law PY, Wessendorf MW (1995) The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci U S A 92:5062–5066. 10.1073/pnas.92.11.5062 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
