S1P/S1P Receptor Signaling in Neuromuscolar Disorders
- PMID: 31861214
- PMCID: PMC6941007
- DOI: 10.3390/ijms20246364
S1P/S1P Receptor Signaling in Neuromuscolar Disorders
Abstract
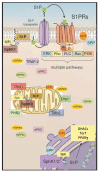
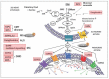
The bioactive sphingolipid metabolite, sphingosine 1-phosphate (S1P), and the signaling pathways triggered by its binding to specific G protein-coupled receptors play a critical regulatory role in many pathophysiological processes, including skeletal muscle and nervous system degeneration. The signaling transduced by S1P binding appears to be much more complex than previously thought, with important implications for clinical applications and for personalized medicine. In particular, the understanding of S1P/S1P receptor signaling functions in specific compartmentalized locations of the cell is worthy of being better investigated, because in various circumstances it might be crucial for the development or/and the progression of neuromuscular diseases, such as Charcot-Marie-Tooth disease, myasthenia gravis, and Duchenne muscular dystrophy.
Keywords: Charcot-Marie-Tooth disease; ceramide; duchenne muscular dystrophy; myasthenia gravis; nervous system; neuromuscular disease; skeletal muscle; sphingolipids; sphingosine 1-phosphate receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway.PLoS One. 2012;7(5):e37218. doi: 10.1371/journal.pone.0037218. Epub 2012 May 14. PLoS One. 2012. PMID: 22606352 Free PMC article.
-
Molecular mechanism of sphingosine-1-phosphate action in Duchenne muscular dystrophy.Dis Model Mech. 2014 Jan;7(1):41-54. doi: 10.1242/dmm.013631. Epub 2013 Sep 25. Dis Model Mech. 2014. PMID: 24077965 Free PMC article.
-
Sphingosine-1-phosphate signaling in blood pressure regulation.Am J Physiol Renal Physiol. 2019 Sep 1;317(3):F638-F640. doi: 10.1152/ajprenal.00572.2018. Epub 2019 Aug 7. Am J Physiol Renal Physiol. 2019. PMID: 31390266 Free PMC article. Review.
-
Sphingosine 1-Phosphate (S1P)/ S1P Receptor Signaling and Mechanotransduction: Implications for Intrinsic Tissue Repair/Regeneration.Int J Mol Sci. 2019 Nov 7;20(22):5545. doi: 10.3390/ijms20225545. Int J Mol Sci. 2019. PMID: 31703256 Free PMC article. Review.
-
The role of sphingosine-1-phosphate in skeletal muscle: Physiology, mechanisms, and clinical perspectives.J Cell Physiol. 2019 Jul;234(7):10047-10059. doi: 10.1002/jcp.27870. Epub 2018 Dec 6. J Cell Physiol. 2019. PMID: 30523638 Review.
Cited by
-
Trends in the Use of Sphingosine 1 Phosphate in Age-Related Diseases: A Scientometric Research Study (1992-2020).J Diabetes Res. 2021 Feb 25;2021:4932974. doi: 10.1155/2021/4932974. eCollection 2021. J Diabetes Res. 2021. PMID: 33791388 Free PMC article.
-
Discovery of a Promising Fluorine-18 Positron Emission Tomography Radiotracer for Imaging Sphingosine-1-Phosphate Receptor 1 in the Brain.J Med Chem. 2023 Apr 13;66(7):4671-4688. doi: 10.1021/acs.jmedchem.2c01752. Epub 2023 Mar 16. J Med Chem. 2023. PMID: 36926861 Free PMC article.
-
S1P Increases VEGF Production in Osteoblasts and Facilitates Endothelial Progenitor Cell Angiogenesis by Inhibiting miR-16-5p Expression via the c-Src/FAK Signaling Pathway in Rheumatoid Arthritis.Cells. 2021 Aug 23;10(8):2168. doi: 10.3390/cells10082168. Cells. 2021. PMID: 34440937 Free PMC article.
-
SARS-CoV-2 Infection: A Role for S1P/S1P Receptor Signaling in the Nervous System?Int J Mol Sci. 2020 Sep 15;21(18):6773. doi: 10.3390/ijms21186773. Int J Mol Sci. 2020. PMID: 32942748 Free PMC article.
-
Sphingosine 1-phosphate Receptor Modulator Therapy for Multiple Sclerosis: Differential Downstream Receptor Signalling and Clinical Profile Effects.Drugs. 2021 Feb;81(2):207-231. doi: 10.1007/s40265-020-01431-8. Drugs. 2021. PMID: 33289881 Free PMC article. Review.
References
-
- Loh K.C., Leong W.I., Carlson M.E., Oskouian B., Kumar A., Fyrst H., Zhang M., Proia R.L., Hoffman E.P., Saba J.D. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS ONE. 2012;7:e37218. doi: 10.1371/annotation/7e7ac57d-30ae-4e49-9138-e3bdbe3491d2. - DOI - PMC - PubMed
-
- Ieronimakis N., Pantoja M., Hays A.L., Dosey T.L., Qi J., Fischer K.A., Hoofnagle A.N., Sadilek M., Chamberlain J.S., Ruohola-Baker H., et al. Increased sphingosine-1-phosphate improves muscle regeneration in acutely injured mdx mice. Skelet. Muscle. 2013;3:20. doi: 10.1186/2044-5040-3-20. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

