An Overview of the Intrinsic Role of Citrullination in Autoimmune Disorders
- PMID: 31886309
- PMCID: PMC6899306
- DOI: 10.1155/2019/7592851
An Overview of the Intrinsic Role of Citrullination in Autoimmune Disorders
Abstract
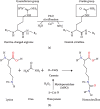
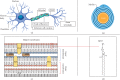
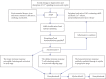
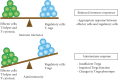
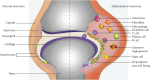
A protein undergoes many types of posttranslation modification. Citrullination is one of these modifications, where an arginine amino acid is converted to a citrulline amino acid. This process depends on catalytic enzymes such as peptidylarginine deiminase enzymes (PADs). This modification leads to a charge shift, which affects the protein structure, protein-protein interactions, and hydrogen bond formation, and it may cause protein denaturation. The irreversible citrullination reaction is not limited to a specific protein, cell, or tissue. It can _target a wide range of proteins in the cell membrane, cytoplasm, nucleus, and mitochondria. Citrullination is a normal reaction during cell death. Apoptosis is normally accompanied with a clearance process via scavenger cells. A defect in the clearance system either in terms of efficiency or capacity may occur due to massive cell death, which may result in the accumulation and leakage of PAD enzymes and the citrullinated peptide from the necrotized cell which could be recognized by the immune system, where the immunological tolerance will be avoided and the autoimmune disorders will be subsequently triggered. The induction of autoimmune responses, autoantibody production, and cytokines involved in the major autoimmune diseases will be discussed.
Copyright © 2019 Mohammed Alghamdi et al.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Citrullination and PAD Enzyme Biology in Type 1 Diabetes - Regulators of Inflammation, Autoimmunity, and Pathology.Front Immunol. 2021 Jun 1;12:678953. doi: 10.3389/fimmu.2021.678953. eCollection 2021. Front Immunol. 2021. PMID: 34140951 Free PMC article. Review.
-
An interplay of structure and intrinsic disorder in the functionality of peptidylarginine deiminases, a family of key autoimmunity-related enzymes.Cell Mol Life Sci. 2019 Dec;76(23):4635-4662. doi: 10.1007/s00018-019-03237-8. Epub 2019 Jul 24. Cell Mol Life Sci. 2019. PMID: 31342121 Free PMC article. Review.
-
Citrullination: A modification important in the pathogenesis of autoimmune diseases.Clin Immunol. 2022 Dec;245:109134. doi: 10.1016/j.clim.2022.109134. Epub 2022 Sep 30. Clin Immunol. 2022. PMID: 36184053 Review.
-
Peptidylarginine deiminase enzymes and citrullinated proteins in female reproductive physiology and associated diseases†.Biol Reprod. 2022 Dec 10;107(6):1395-1410. doi: 10.1093/biolre/ioac173. Biol Reprod. 2022. PMID: 36087287 Free PMC article. Review.
-
Post-translational modifications such as citrullination are excellent _targets for cancer therapy.Semin Immunol. 2020 Feb;47:101393. doi: 10.1016/j.smim.2020.101393. Epub 2020 Jan 10. Semin Immunol. 2020. PMID: 31932199 Review.
Cited by
-
L-Citrullinato-Bipyridine and L-Citrullinato-Phenanthroline Mixed Copper Complexes: Synthesis, Characterization and Potential Anticancer Activity.Pharmaceutics. 2024 May 31;16(6):747. doi: 10.3390/pharmaceutics16060747. Pharmaceutics. 2024. PMID: 38931869 Free PMC article.
-
Autoantigen Discovery in the Hair Loss Disorder, Alopecia Areata: Implication of Post-Translational Modifications.Front Immunol. 2022 Jun 3;13:890027. doi: 10.3389/fimmu.2022.890027. eCollection 2022. Front Immunol. 2022. PMID: 35720384 Free PMC article. Review.
-
Brain-Region-Specific Differences in Protein Citrullination/Deimination in a Pre-Motor Parkinson's Disease Rat Model.Int J Mol Sci. 2024 Oct 17;25(20):11168. doi: 10.3390/ijms252011168. Int J Mol Sci. 2024. PMID: 39456949 Free PMC article.
-
Post-Translational Modifications in Tumor-Associated Antigens as a Platform for Novel Immuno-Oncology Therapies.Cancers (Basel). 2022 Dec 26;15(1):138. doi: 10.3390/cancers15010138. Cancers (Basel). 2022. PMID: 36612133 Free PMC article. Review.
-
Impact of Posttranslational Modification in Pathogenesis of Rheumatoid Arthritis: Focusing on Citrullination, Carbamylation, and Acetylation.Int J Mol Sci. 2021 Sep 30;22(19):10576. doi: 10.3390/ijms221910576. Int J Mol Sci. 2021. PMID: 34638916 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

