Lysosomal Fusion: An Efficient Mechanism Increasing Their Sequestration Capacity for Weak Base Drugs without Apparent Lysosomal Biogenesis
- PMID: 31947839
- PMCID: PMC7022710
- DOI: 10.3390/biom10010077
Lysosomal Fusion: An Efficient Mechanism Increasing Their Sequestration Capacity for Weak Base Drugs without Apparent Lysosomal Biogenesis
Abstract
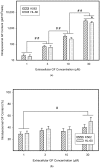
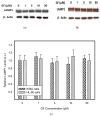
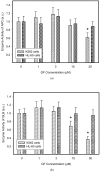
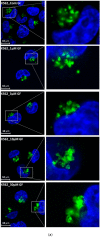
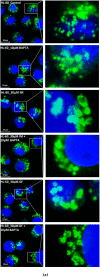
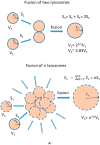
Lysosomal sequestration of anticancer therapeutics lowers their cytotoxic potential, reduces drug availability at _target sites, and contributes to cancer resistance. Only recently has it been shown that lysosomal sequestration of weak base drugs induces lysosomal biogenesis mediated by activation of transcription factor EB (TFEB) which, in turn, enhances their accumulation capacity, thereby increasing resistance to these drugs. Here, we addressed the question of whether lysosomal biogenesis is the only mechanism that increases lysosomal sequestration capacity. We found that lysosomal sequestration of some tyrosine kinase inhibitors (TKIs), gefitinib (GF) and imatinib (IM), induced expansion of the lysosomal compartment. However, an expression analysis of lysosomal genes, including lysosome-associated membrane proteins 1, 2 (LAMP1, LAMP2), vacuolar ATPase subunit B2 (ATP6V1B2), acid phosphatase (ACP), and galactosidase beta (GLB) controlled by TFEB, did not reveal increased expression. Instead, we found that both studied TKIs, GF and IM, induced lysosomal fusion which was dependent on nicotinic acid adenine dinucleotide phosphate (NAADP) mediated Ca2+signaling. A theoretical analysis revealed that lysosomal fusion is sufficient to explain the enlargement of lysosomal sequestration capacity. In conclusion, we demonstrated that extracellular TKIs, GF and IM, induced NAADP/Ca2+ mediated lysosomal fusion, leading to enlargement of the lysosomal compartment with significantly increased sequestration capacity for these drugs without apparent lysosomal biogenesis.
Keywords: Hl-60 cells; K562 cells; lysosomal fusion; lysosomal sequestration capacity; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











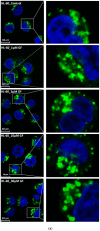



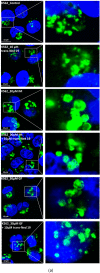

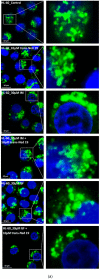

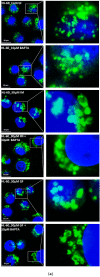



Similar articles
-
Changes in expression of lysosomal membrane proteins in leucocytes of cancer patients treated with tyrosine kinase inhibitors.Cancer Chemother Pharmacol. 2021 Jul;88(1):89-98. doi: 10.1007/s00280-021-04266-6. Epub 2021 Mar 30. Cancer Chemother Pharmacol. 2021. PMID: 33783548
-
The Lysosomal Sequestration of Tyrosine Kinase Inhibitors and Drug Resistance.Biomolecules. 2019 Oct 31;9(11):675. doi: 10.3390/biom9110675. Biomolecules. 2019. PMID: 31683643 Free PMC article.
-
Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance.Onco_target. 2015 Jan 20;6(2):1143-56. doi: 10.18632/onco_target.2732. Onco_target. 2015. PMID: 25544758 Free PMC article.
-
Lysosomes as mediators of drug resistance in cancer.Drug Resist Updat. 2016 Jan;24:23-33. doi: 10.1016/j.drup.2015.11.004. Epub 2015 Nov 26. Drug Resist Updat. 2016. PMID: 26830313 Review.
-
The role of endolysosomal trafficking in anticancer drug resistance.Drug Resist Updat. 2021 Jul;57:100769. doi: 10.1016/j.drup.2021.100769. Epub 2021 Jun 2. Drug Resist Updat. 2021. PMID: 34217999 Review.
Cited by
-
Lysosomal Biogenesis and Implications for Hydroxychloroquine Disposition.J Pharmacol Exp Ther. 2021 Feb;376(2):294-305. doi: 10.1124/jpet.120.000309. Epub 2020 Nov 10. J Pharmacol Exp Ther. 2021. PMID: 33172973 Free PMC article.
-
Endolysosomal TRPMLs in Cancer.Biomolecules. 2021 Jan 6;11(1):65. doi: 10.3390/biom11010065. Biomolecules. 2021. PMID: 33419007 Free PMC article. Review.
-
What Is the Significance of Lysosomal-Mediated Resistance to Imatinib?Cells. 2023 Feb 23;12(5):709. doi: 10.3390/cells12050709. Cells. 2023. PMID: 36899844 Free PMC article. Review.
References
-
- De Duve C. Lysosomes, a new group of cytoplasmic particles. In: Hayashi T., editor. Subcellular Particles. The Ronald Press Co.; New York, NY, USA: 1959. pp. 128–159.
-
- Lűllmann-Rauch R. History and morphology of the lysosome. In: Saftig P., editor. Lysosomes. Springer; New York, NY, USA: 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

