Clonally selected primitive endothelial cells promote occlusive pulmonary arteriopathy and severe pulmonary hypertension in rats exposed to chronic hypoxia
- PMID: 31980720
- PMCID: PMC6981224
- DOI: 10.1038/s41598-020-58083-7
Clonally selected primitive endothelial cells promote occlusive pulmonary arteriopathy and severe pulmonary hypertension in rats exposed to chronic hypoxia
Abstract
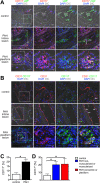
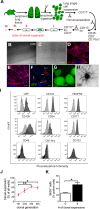
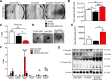
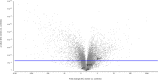
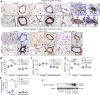
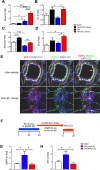
One current concept suggests that unchecked proliferation of clonally selected precursors of endothelial cells (ECs) contribute to severe pulmonary arterial hypertension (PAH). We hypothesized that clonally selected ECs expressing the progenitor marker CD117 promote severe occlusive pulmonary hypertension (PH). The remodelled pulmonary arteries of PAH patients harboured CD117+ ECs. Rat lung CD117+ ECs underwent four generations of clonal expansion to enrich hyperproliferative ECs. The resulting clonally enriched ECs behaved like ECs, as measured by in vitro and in vivo angiogenesis assays. The same primitive ECs showed a limited ability for mesenchymal lineage differentiation. Endothelial differentiation and function were enhanced by blocking TGF-β signalling, promoting bone morphogenic protein (BMP) signalling. The transplantation of the EC clones caused arterio-occlusive PH in rats exposed to chronic hypoxia. These EC clones engrafted in the pulmonary arteries. Yet cessation of chronic hypoxia promoted lung cell apoptosis and resolution of vascular lesions. In conclusion, this is to the best of our knowledge, the first report that clonally enriched primitive ECs promote occlusive pulmonary arteriopathy and severe PH. These primitive EC clones further give rise to cells of endothelial and mesenchymal lineage as directed by BMP and TGF-β signaling.
Conflict of interest statement
M.K. declares grants from Prometic, grants and personal fees from Roche, Boehringer Ingelheim, GSK, Gilead, Actelion, Respivert, personal fees from Genoa, grants from Alkermes, grants from Pharmaxis, outside the submitted work. The other authors have no competing interests.
Figures

Similar articles
-
Endothelial Heterogeneity in the Response to Autophagy Drives Small Vessel Muscularization in Pulmonary Hypertension.Circulation. 2024 Aug 6;150(6):466-487. doi: 10.1161/CIRCULATIONAHA.124.068726. Epub 2024 Jun 14. Circulation. 2024. PMID: 38873770
-
Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension.Circ Res. 2006 Feb 3;98(2):209-17. doi: 10.1161/01.RES.0000200180.01710.e6. Epub 2005 Dec 15. Circ Res. 2006. PMID: 16357305
-
Endothelial cell-related autophagic pathways in Sugen/hypoxia-exposed pulmonary arterial hypertensive rats.Am J Physiol Lung Cell Mol Physiol. 2017 Nov 1;313(5):L899-L915. doi: 10.1152/ajplung.00527.2016. Epub 2017 Aug 10. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28798259
-
Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials.Pharmacol Ther. 2010 Apr;126(1):1-8. doi: 10.1016/j.pharmthera.2009.12.006. Epub 2010 Feb 1. Pharmacol Ther. 2010. PMID: 20117135 Review.
-
Estrogens and development of pulmonary hypertension: interaction of estradiol metabolism and pulmonary vascular disease.J Cardiovasc Pharmacol. 2010 Dec;56(6):696-708. doi: 10.1097/FJC.0b013e3181f9ea8d. J Cardiovasc Pharmacol. 2010. PMID: 20881610 Free PMC article. Review.
Cited by
-
Endothelial cell clonality, heterogeneity and dysfunction in pulmonary arterial hypertension.Front Med (Lausanne). 2023 Dec 6;10:1304766. doi: 10.3389/fmed.2023.1304766. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38126077 Free PMC article. Review.
-
When Innate Immunity Meets Angiogenesis-The Role of Toll-Like Receptors in Endothelial Cells and Pulmonary Hypertension.Front Med (Lausanne). 2020 Jul 31;7:352. doi: 10.3389/fmed.2020.00352. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32850883 Free PMC article. Review.
-
Progenitor/Stem Cells in Vascular Remodeling during Pulmonary Arterial Hypertension.Cells. 2021 May 28;10(6):1338. doi: 10.3390/cells10061338. Cells. 2021. PMID: 34071347 Free PMC article. Review.
-
Immature reticulocyte fraction: A novel biomarker of hemodynamic severity in pulmonary arterial hypertension.Pulm Circ. 2024 Aug 5;14(3):e12421. doi: 10.1002/pul2.12421. eCollection 2024 Jul. Pulm Circ. 2024. PMID: 39105130 Free PMC article.
-
A p53-TLR3 axis ameliorates pulmonary hypertension by inducing BMPR2 via IRF3.iScience. 2023 Jan 5;26(2):105935. doi: 10.1016/j.isci.2023.105935. eCollection 2023 Feb 17. iScience. 2023. PMID: 36685041 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

