Soluble Fas affects erythropoiesis in vitro and acts as a potential predictor of erythropoiesis-stimulating agent therapy in patients with chronic kidney disease
- PMID: 32003597
- PMCID: PMC7474254
- DOI: 10.1152/ajprenal.00433.2019
Soluble Fas affects erythropoiesis in vitro and acts as a potential predictor of erythropoiesis-stimulating agent therapy in patients with chronic kidney disease
Abstract
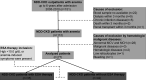
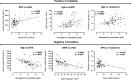
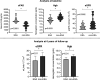
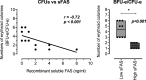
Serum soluble Fas (sFas) levels are associated with erythropoietin (Epo) hyporesponsiveness in patients with chronic kidney disease (CKD). Whether sFas could predict the need for erythropoiesis-stimulating agent (ESA) usage and its influence in erythropoiesis remain unclear. We evaluated the relation between sFas and ESA therapy in patients with CKD with anemia and its effect on erythropoiesis in vitro. First, we performed a retrospective cohort study with 77 anemic patients with nondialysis CKD. We performed in vitro experiments to investigate whether sFas could interfere with the behavior of hematopoietic stem cells (HSCs). HSCs were isolated from umbilical cord blood and incubated with recombinant sFas protein in a dose-dependent manner. Serum sFas positively correlated with Epo levels (r = 0.30, P = 0.001) but negatively with hemoglobin (r = -0.55, P < 0.001) and glomerular filtration rate (r = -0.58, P < 0.001) in patients with CKD at baseline. Elevated sFas serum levels (4,316 ± 897 vs. 2,776 ± 749, P < 0.001) with lower estimated glomerular filtration rate (26.2 ± 10.1 vs. 33.5 ± 14.3, P = 0.01) and reduced hemoglobin concentration (11.1 ± 0.9 vs. 12.5 ± 1.2, P < 0.001) were identified in patients who required ESA therapy compared with patients with non-ESA. Afterward, we detected that the sFas level was slight correlated with a necessity of ESA therapy in patients with nondialysis CKD and anemia. In vitro assays demonstrated that the erythroid progenitor cell frequency negatively correlated with sFas concentration (r = -0.72, P < 0.001). There was decreased erythroid colony formation in vitro when CD34+ HSCs were incubated with a higher concentration of sFas protein (1.56 ± 0.29, 4.33 ± 0.53, P < 0.001). Our findings suggest that sFas is a potential predictor for ESA therapy in patients with nondialysis CKD and that elevated sFas could affect erythropoiesis in vitro.
Keywords: anemia and hematopoietic stem cells; chronic kidney disease; erythropoiesis-stimulating agents; soluble Fas.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures

Similar articles
-
In search of potential predictors of erythropoiesis-stimulating agents (ESAs) hyporesponsiveness: a population-based study.BMC Nephrol. 2019 Sep 14;20(1):359. doi: 10.1186/s12882-019-1554-0. BMC Nephrol. 2019. PMID: 31521117 Free PMC article.
-
Rationale and design of oBservational clinical Research In chronic kidney disease patients with renal anemia: renal proGnosis in patients with Hyporesponsive anemia To Erythropoiesis-stimulating agents, darbepoetiN alfa (BRIGHTEN Trial).Clin Exp Nephrol. 2018 Feb;22(1):78-84. doi: 10.1007/s10157-017-1427-4. Epub 2017 Jun 28. Clin Exp Nephrol. 2018. PMID: 28660446 Free PMC article.
-
A randomized, placebo-controlled trial of pentoxifylline on erythropoiesis-stimulating agent hyporesponsiveness in anemic patients with CKD: the Handling Erythropoietin Resistance With Oxpentifylline (HERO) trial.Am J Kidney Dis. 2015 Jan;65(1):49-57. doi: 10.1053/j.ajkd.2014.06.020. Epub 2014 Aug 10. Am J Kidney Dis. 2015. PMID: 25115616 Clinical Trial.
-
Iron Metabolism in Chronic Kidney Disease Patients.Contrib Nephrol. 2019;198:103-111. doi: 10.1159/000496369. Epub 2019 Apr 16. Contrib Nephrol. 2019. PMID: 30991401 Review.
-
The Evolution of _target Hemoglobin Levels in Anemia of Chronic Kidney Disease.Adv Chronic Kidney Dis. 2019 Jul;26(4):229-236. doi: 10.1053/j.ackd.2019.06.001. Adv Chronic Kidney Dis. 2019. PMID: 31477253 Review.
Cited by
-
Relationship of hemoglobin levels with outcomes in deceased donor kidney transplant: a retrospective cohort study.J Bras Nefrol. 2024 Apr-Jun;46(2):e20230014. doi: 10.1590/2175-8239-JBN-2023-0014en. J Bras Nefrol. 2024. PMID: 38284551 Free PMC article.
-
Baseline Ratio of Soluble Fas/FasL Predicts Onset of Pulmonary Hypertension in Elder Patients Undergoing Maintenance Hemodialysis: A Prospective Cohort Study.Front Physiol. 2022 Mar 1;13:847172. doi: 10.3389/fphys.2022.847172. eCollection 2022. Front Physiol. 2022. PMID: 35299658 Free PMC article.
-
A retrospective view of the relationship of soluble Fas with anemia and outcomes in chronic kidney disease.PLoS One. 2023 Jun 30;18(6):e0286854. doi: 10.1371/journal.pone.0286854. eCollection 2023. PLoS One. 2023. PMID: 37390095 Free PMC article.
-
Acute kidney injury: Incidence, risk factors, and outcomes in severe COVID-19 patients.PLoS One. 2021 May 25;16(5):e0251048. doi: 10.1371/journal.pone.0251048. eCollection 2021. PLoS One. 2021. PMID: 34033655 Free PMC article.
References
-
- Blaes J, Thomé CM, Pfenning PN, Rübmann P, Sahm F, Wick A, Bunse T, Schmenger T, Sykora J, von Deimling A, Wiestler B, Merz C, Jugold M, Haberkorn U, Abdollahi A, Debus J, Gieffers C, Kunz C, Bendszus M, Kluge M, Platten M, Fricke H, Wick W, Lemke D. Inhibition of CD95/CD95L (FAS/FASLG) signaling with APG101 prevents invasion and enhances radiation therapy for glioblastoma. Mol Cancer Res 16: 767–776, 2018. doi:10.1158/1541-7786.MCR-17-0563. - DOI - PubMed
-
- Bhatraju PK, Robinson-Cohen C, Mikacenic C, Harju-Baker S, Dmyterko V, Slivinski NSJ, Liles WC, Himmelfarb J, Heckbert SR, Wurfel MM. Circulating levels of soluble Fas (sCD95) are associated with risk for development of a nonresolving acute kidney injury subphenotype. Crit Care 21: 217, 2017. doi:10.1186/s13054-017-1807-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

