Microglia in the hypothalamus respond to tumor-derived factors and are protective against cachexia during pancreatic cancer
- PMID: 32039522
- PMCID: PMC7205589
- DOI: 10.1002/glia.23796
Microglia in the hypothalamus respond to tumor-derived factors and are protective against cachexia during pancreatic cancer
Abstract
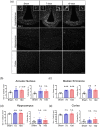
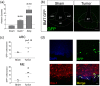
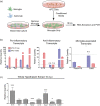
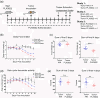
Microglia in the mediobasal hypothalamus (MBH) respond to inflammatory stimuli and metabolic perturbations to mediate body composition. This concept is well studied in the context of high fat diet induced obesity (HFDO), yet has not been investigated in the context of cachexia, a devastating metabolic syndrome characterized by anorexia, fatigue, and muscle catabolism. We show that microglia accumulate specifically in the MBH early in pancreatic ductal adenocarcinoma (PDAC)-associated cachexia and assume an activated morphology. Furthermore, we observe astrogliosis in the MBH and hippocampus concurrent with cachexia initiation. We next show that circulating immune cells resembling macrophages infiltrate the MBH. PDAC-derived factors induced microglia to express a transcriptional profile in vitro that was distinct from that induced by lipopolysaccharide (LPS). Microglia depletion through CSF1-R antagonism resulted in accelerated cachexia onset and increased anorexia, fatigue, and muscle catabolism during PDAC. This corresponded with increased hypothalamic-pituitary-adrenal (HPA) axis activation. CSF1-R antagonism had little effect on inflammatory response in the circulation, liver, or tumor. These findings demonstrate that microglia are protective against PDAC cachexia and provide mechanistic insight into this function.
Keywords: brain; cachexia; hypothalamus; microglia; neuroinflammation; pancreatic cancer.
© 2020 The Authors. Glia published by Wiley Periodicals, Inc.
Figures


Similar articles
-
Critical changes in hypothalamic gene networks in response to pancreatic cancer as found by single-cell RNA sequencing.Mol Metab. 2022 Apr;58:101441. doi: 10.1016/j.molmet.2022.101441. Epub 2022 Jan 11. Mol Metab. 2022. PMID: 35031523 Free PMC article.
-
Macrophages potentiate STAT3 signaling in skeletal muscles and regulate pancreatic cancer cachexia.Cancer Lett. 2020 Aug 1;484:29-39. doi: 10.1016/j.canlet.2020.04.017. Epub 2020 Apr 25. Cancer Lett. 2020. PMID: 32344015 Free PMC article.
-
Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function.Cell Rep. 2014 Dec 24;9(6):2124-38. doi: 10.1016/j.celrep.2014.11.018. Epub 2014 Dec 11. Cell Rep. 2014. PMID: 25497089 Free PMC article.
-
Metabolic factors in the regulation of hypothalamic innate immune responses in obesity.Exp Mol Med. 2022 Apr;54(4):393-402. doi: 10.1038/s12276-021-00666-z. Epub 2022 Apr 26. Exp Mol Med. 2022. PMID: 35474339 Free PMC article. Review.
-
The role of hypothalamic inflammation, the hypothalamic-pituitary-adrenal axis and serotonin in the cancer anorexia-cachexia syndrome.Curr Opin Clin Nutr Metab Care. 2017 Sep;20(5):396-401. doi: 10.1097/MCO.0000000000000401. Curr Opin Clin Nutr Metab Care. 2017. PMID: 28708669 Review.
Cited by
-
Circulating myeloid cells invade the central nervous system to mediate cachexia during pancreatic cancer.Elife. 2020 May 11;9:e54095. doi: 10.7554/eLife.54095. Elife. 2020. PMID: 32391790 Free PMC article.
-
Physiologic and molecular characterization of a novel murine model of metastatic head and neck cancer cachexia.J Cachexia Sarcopenia Muscle. 2021 Oct;12(5):1312-1332. doi: 10.1002/jcsm.12745. Epub 2021 Jul 6. J Cachexia Sarcopenia Muscle. 2021. PMID: 34231343 Free PMC article.
-
Lipopolysaccharide-induced hypothalamic inflammation in cancer cachexia-anorexia is amplified by tumour-derived prostaglandin E2.J Cachexia Sarcopenia Muscle. 2022 Dec;13(6):3014-3027. doi: 10.1002/jcsm.13093. Epub 2022 Oct 27. J Cachexia Sarcopenia Muscle. 2022. PMID: 36303458 Free PMC article.
-
The roles of P-selectin in cancer cachexia.Med Oncol. 2023 Oct 23;40(11):338. doi: 10.1007/s12032-023-02207-2. Med Oncol. 2023. PMID: 37870739 Review.
-
Chronic cerebral lipocalin 2 exposure elicits hippocampal neuronal dysfunction and cognitive impairment.Brain Behav Immun. 2021 Oct;97:102-118. doi: 10.1016/j.bbi.2021.07.002. Epub 2021 Jul 8. Brain Behav Immun. 2021. PMID: 34245812 Free PMC article.
References
-
- Bossy‐Wetzel, E. , Talantova, M. V. , Lee, W. D. , Schölzke, M. N. , Harrop, A. , Mathews, E. , … Lipton, S. A. (2004). Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38‐activated K+ channels. Neuron, 41(3), 351–365. 10.1016/S0896-6273(04)00015-7 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

