Impact of Mesenchymal Stromal Cells and Their Extracellular Vesicles in a Rat Model of Kidney Rejection
- PMID: 32064259
- PMCID: PMC7000363
- DOI: 10.3389/fcell.2020.00010
Impact of Mesenchymal Stromal Cells and Their Extracellular Vesicles in a Rat Model of Kidney Rejection
Abstract
Background: Mesenchymal stromal cells (MSCs) from different sources possess great therapeutic potential due to their immunomodulatory properties associated with allograft tolerance. However, a crucial role in this activity resides in extracellular vesicles (EVs) and signaling molecules secreted by cells. This study aimed to evaluate the immunomodulatory properties of donor and recipient MSCs isolated from adipose tissue (AD) or bone marrow (BM) and their EVs on kidney outcome in a rat kidney transplant model.
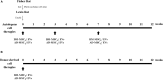
Methods: The heterotopic-kidney-transplant Fisher-to-Lewis rat model (F-L) was performed to study mixed cellular and humoral rejection. After kidney transplantation, Lewis recipients were assigned to 10 groups; two control groups; four groups received autologous MSCs (either AD- or BM- MSC) or EVs (derived from both cell types); and four groups received donor-derived MSCs or EVs. AD and BM-EVs were purified by ultracentrifugation. Autologous cell therapies were administered three times intravenously; immediately after kidney transplantation, 4 and 8 weeks, whereas donor-derived cell therapies were administered once intravenously immediately after transplantation. Survival and renal function were monitored. Twelve weeks after kidney transplantation grafts were harvested, infiltrating lymphocytes were analyzed by flow cytometry and histological lesions were characterized.
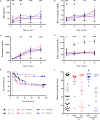
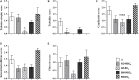
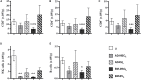
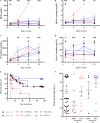
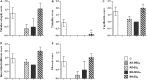
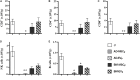
Results: Autologous AD- and BM-MSCs, but not their EVs, prolonged graft and recipient survival in a rat model of kidney rejection. Autologous AD- and BM-MSCs significantly improved renal function during the first 4 weeks after transplantation. The amelioration of graft function could be associated with an improvement in tubular damage, as well as in T, and NK cell infiltration. On the other side, the application of donor-derived AD-MSC was harmful, and all rats died before the end of the protocol. AD-EVs did not accelerate the rejection. Contrary to autologous MSCs results, the single dose of donor-derived BM-MSCs is not enough to ameliorate kidney graft damage.
Conclusion: EVs treatments did not exert any benefit in our experimental settings. In the autologous setting, BM-MSCs prompted as a potentially promising therapy to improve kidney graft outcomes in rats with chronic mixed rejection. In the donor-derived setting, AD-MSC accelerated progression to end-stage kidney disease. Further experiments are required to adjust timing and dose for better long-term outcomes.
Keywords: adipose tissue; bone marrow; chronic kidney disease; extracellular vesicles; immunomodulation; kidney transplantation; mesenchymal stromal cells.
Copyright © 2020 Ramirez-Bajo, Rovira, Lazo-Rodriguez, Banon-Maneus, Tubita, Moya-Rull, Hierro-Garcia, Ventura-Aguiar, Oppenheimer, Campistol and Diekmann.
Figures
Similar articles
-
Paracrine Proangiogenic Function of Human Bone Marrow-Derived Mesenchymal Stem Cells Is Not Affected by Chronic Kidney Disease.Stem Cells Int. 2019 Dec 23;2019:1232810. doi: 10.1155/2019/1232810. eCollection 2019. Stem Cells Int. 2019. PMID: 31933648 Free PMC article.
-
Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations.Stem Cells. 2018 Mar;36(3):434-445. doi: 10.1002/stem.2759. Epub 2017 Dec 27. Stem Cells. 2018. PMID: 29239062
-
Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells Prolong Graft Survival in Vascularized Composite Allotransplantation.Transplantation. 2015 Sep;99(9):1765-73. doi: 10.1097/TP.0000000000000731. Transplantation. 2015. PMID: 26102613
-
Multipotent mesenchymal stromal cells in kidney transplant recipients: The next big thing?Blood Rev. 2021 Jan;45:100718. doi: 10.1016/j.blre.2020.100718. Epub 2020 May 29. Blood Rev. 2021. PMID: 32507576 Review.
-
Mesenchymal stromal cells in renal transplantation: opportunities and challenges.Nat Rev Nephrol. 2016 Apr;12(4):241-53. doi: 10.1038/nrneph.2016.7. Epub 2016 Feb 8. Nat Rev Nephrol. 2016. PMID: 26853275 Review.
Cited by
-
Adipose-Derived Stem/Stromal Cells in Kidney Transplantation: Status Quo and Future Perspectives.Int J Mol Sci. 2021 Oct 17;22(20):11188. doi: 10.3390/ijms222011188. Int J Mol Sci. 2021. PMID: 34681848 Free PMC article. Review.
-
Emerging Role and Mechanism of Mesenchymal Stem Cells-Derived Extracellular Vesicles in Rheumatic Disease.J Inflamm Res. 2024 Sep 30;17:6827-6846. doi: 10.2147/JIR.S488201. eCollection 2024. J Inflamm Res. 2024. PMID: 39372581 Free PMC article. Review.
-
Toward transplantation tolerance with adipose tissue-derived therapeutics.Front Immunol. 2023 Apr 28;14:1111813. doi: 10.3389/fimmu.2023.1111813. eCollection 2023. Front Immunol. 2023. PMID: 37187733 Free PMC article. Review.
-
Exosomes as New Biomarkers and Drug Delivery Tools for the Prevention and Treatment of Various Diseases: Current Perspectives.Int J Mol Sci. 2021 Jul 21;22(15):7763. doi: 10.3390/ijms22157763. Int J Mol Sci. 2021. PMID: 34360530 Free PMC article. Review.
-
Isolation of Extracellular Vesicles Derived from Mesenchymal Stromal Cells by Ultracentrifugation.Bio Protoc. 2020 Dec 20;10(24):e3860. doi: 10.21769/BioProtoc.3860. eCollection 2020 Dec 20. Bio Protoc. 2020. PMID: 33855106 Free PMC article.
References
-
- Alvaro-Gracia J. M., Jover J. A., Garcia-Vicuna R., Carreno L., Alonso A., Marsal S., et al. (2017). Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 76 196–202. 10.1136/annrheumdis-2015-208918 - DOI - PubMed
-
- Ammar H. I., Sequiera G. L., Nashed M. B., Ammar R. I., Gabr H. M., Elsayed H. E., et al. (2015). Comparison of adipose tissue- and bone marrow- derived mesenchymal stem cells for alleviating doxorubicin-induced cardiac dysfunction in diabetic rats. Stem Cell Res. Ther. 6:148. 10.1186/s13287-015-0142-x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

