Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology
- PMID: 32079324
- PMCID: PMC7072240
- DOI: 10.3390/biom10020320
Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology
Abstract
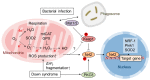
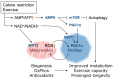
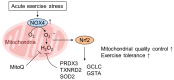
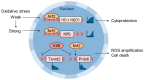
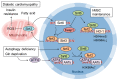
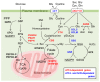
Reactive oxygen species (ROS) are byproducts of aerobic respiration and signaling molecules that control various cellular functions. Nrf2 governs the gene expression of endogenous antioxidant synthesis and ROS-eliminating enzymes in response to various electrophilic compounds that inactivate the negative regulator Keap1. Accumulating evidence has shown that mitochondrial ROS (mtROS) activate Nrf2, often mediated by certain protein kinases, and induce the expression of antioxidant genes and genes involved in mitochondrial quality/quantity control. Mild physiological stress, such as caloric restriction and exercise, elicits beneficial effects through a process known as "mitohormesis." Exercise induces NOX4 expression in the heart, which activates Nrf2 and increases endurance capacity. Mice transiently depleted of SOD2 or overexpressing skeletal muscle-specific UCP1 exhibit Nrf2-mediated antioxidant gene expression and PGC1α-mediated mitochondrial biogenesis. ATF4 activation may induce a transcriptional program that enhances NADPH synthesis in the mitochondria and might cooperate with the Nrf2 antioxidant system. In response to severe oxidative stress, Nrf2 induces Klf9 expression, which represses mtROS-eliminating enzymes to enhance cell death. Nrf2 is inactivated in certain pathological conditions, such as diabetes, but Keap1 down-regulation or mtROS elimination rescues Nrf2 expression and improves the pathology. These reports aid us in understanding the roles of Nrf2 in pathophysiological alterations involving mtROS.
Keywords: ATF4; Keap1; Klf9; Nrf2; PGC1α; Sirt6; mitochondrial ROS.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Angiotensin II induces oxidative stress and upregulates neuroprotective signaling from the NRF2 and KLF9 pathway in dopaminergic cells.Free Radic Biol Med. 2018 Dec;129:394-406. doi: 10.1016/j.freeradbiomed.2018.10.409. Epub 2018 Oct 11. Free Radic Biol Med. 2018. PMID: 30315936
-
The Interplay Between Mitochondrial Reactive Oxygen Species, Endoplasmic Reticulum Stress, and Nrf2 Signaling in Cardiometabolic Health.Antioxid Redox Signal. 2021 Aug 1;35(4):252-269. doi: 10.1089/ars.2020.8220. Epub 2021 Feb 16. Antioxid Redox Signal. 2021. PMID: 33599550 Free PMC article. Review.
-
Nrf2 amplifies oxidative stress via induction of Klf9.Mol Cell. 2014 Mar 20;53(6):916-928. doi: 10.1016/j.molcel.2014.01.033. Epub 2014 Mar 6. Mol Cell. 2014. PMID: 24613345 Free PMC article.
-
Mitochondriotropic antioxidant based on caffeic acid AntiOxCIN4 activates Nrf2-dependent antioxidant defenses and quality control mechanisms to antagonize oxidative stress-induced cell damage.Free Radic Biol Med. 2022 Feb 1;179:119-132. doi: 10.1016/j.freeradbiomed.2021.12.304. Epub 2021 Dec 22. Free Radic Biol Med. 2022. PMID: 34954022
-
Transcriptional activation of antioxidant gene expression by Nrf2 protects against mitochondrial dysfunction and neuronal death associated with acute and chronic neurodegeneration.Exp Neurol. 2020 Jun;328:113247. doi: 10.1016/j.expneurol.2020.113247. Epub 2020 Feb 12. Exp Neurol. 2020. PMID: 32061629 Free PMC article. Review.
Cited by
-
Brazilin prevents against myocardial ischemia-reperfusion injury through the modulation of Nrf2 via the PKC signaling pathway.Ann Transl Med. 2021 Feb;9(4):312. doi: 10.21037/atm-20-4414. Ann Transl Med. 2021. PMID: 33708939 Free PMC article.
-
EVs from BALF-Mediators of Inflammation and Potential Biomarkers in Lung Diseases.Int J Mol Sci. 2021 Apr 1;22(7):3651. doi: 10.3390/ijms22073651. Int J Mol Sci. 2021. PMID: 33915715 Free PMC article. Review.
-
Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation During Infection.Front Immunol. 2020 Aug 14;11:1649. doi: 10.3389/fimmu.2020.01649. eCollection 2020. Front Immunol. 2020. PMID: 32922385 Free PMC article. Review.
-
Comparison of the developmental competence of in vitro-produced mouse embryos cultured under 5 versus 2% O2 with in vivo-derived blastocysts.J Assist Reprod Genet. 2024 Nov;41(11):3089-3103. doi: 10.1007/s10815-024-03267-7. Epub 2024 Sep 23. J Assist Reprod Genet. 2024. PMID: 39313714
-
Peanut Shell Extract Improves Mitochondrial Function in db/db Mice via Suppression of Oxidative Stress and Inflammation.Nutrients. 2024 Jun 21;16(13):1977. doi: 10.3390/nu16131977. Nutrients. 2024. PMID: 38999726 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

