Applying the Host-Microbe Damage Response Framework to Candida Pathogenesis: Current and Prospective Strategies to Reduce Damage
- PMID: 32168864
- PMCID: PMC7151217
- DOI: 10.3390/jof6010035
Applying the Host-Microbe Damage Response Framework to Candida Pathogenesis: Current and Prospective Strategies to Reduce Damage
Abstract
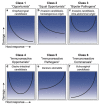
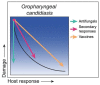
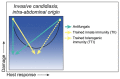
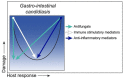
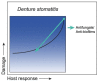
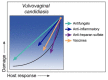
Disease is a complex outcome that can occur as a result of pathogen-mediated damage, host-mediated damage or both. This has led to the revolutionary concept of the damage response framework (DRF) that defines microbial virulence as a function of host immunity. The DRF outlines six scenarios (classes) of host damage or beneficial outcomes, depending on the microbe and the strength of the immune response. Candida albicans is uniquely adapted to its human host and can exist as either a commensal, colonizing various anatomical sites without causing notable damage, or as a pathogen, with the ability to cause a diverse array of diseases, ranging from mucosal to invasive systemic infections that result in varying levels of microbe-mediated and/or host-mediated damage. We recently categorized six different forms of candidiasis (oropharyngeal, hematogenous, intra-abdominal, gastrointestinal, denture stomatitis, and vulvovaginitis) into independent DRF classes, supporting a contemporary view of unique mechanisms of pathogenesis for these Candida infections. In this review, we summarize the evidence for the pathogenesis of these various forms of candidiasis in the context of the DRF with the further intent to provide insights into strategies to achieve a level of host response or outcome otherwise, that limits host damage.
Keywords: Candida albicans; damage response; host; immune response; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Candida albicans Pathogenesis: Fitting within the Host-Microbe Damage Response Framework.Infect Immun. 2016 Sep 19;84(10):2724-39. doi: 10.1128/IAI.00469-16. Print 2016 Oct. Infect Immun. 2016. PMID: 27430274 Free PMC article. Review.
-
New Insights in Candida albicans Innate Immunity at the Mucosa: Toxins, Epithelium, Metabolism, and Beyond.Front Cell Infect Microbiol. 2020 Mar 3;10:81. doi: 10.3389/fcimb.2020.00081. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32195196 Free PMC article. Review.
-
A Simple Nematode Infection Model for Studying Candida albicans Pathogenesis.Curr Protoc Microbiol. 2020 Dec;59(1):e114. doi: 10.1002/cpmc.114. Curr Protoc Microbiol. 2020. PMID: 32975912
-
Candida glabrata Has No Enhancing Role in the Pathogenesis of Candida-Associated Denture Stomatitis in a Rat Model.mSphere. 2019 Apr 3;4(2):e00191-19. doi: 10.1128/mSphere.00191-19. mSphere. 2019. PMID: 30944214 Free PMC article.
-
Intravital Imaging Reveals Divergent Cytokine and Cellular Immune Responses to Candida albicans and Candida parapsilosis.mBio. 2019 May 14;10(3):e00266-19. doi: 10.1128/mBio.00266-19. mBio. 2019. PMID: 31088918 Free PMC article.
Cited by
-
In vitro activities of Traganum nudatum and Mentha pulegium extracts combined with amphotericin B against Candida albicans in production of hydrolytic enzymes.Curr Med Mycol. 2020 Sep;6(3):27-32. doi: 10.18502/cmm.6.3.4499. Curr Med Mycol. 2020. PMID: 33834140 Free PMC article.
-
A protein-free vaccine stimulates innate immunity and protects against nosocomial pathogens.Sci Transl Med. 2023 Oct 4;15(716):eadf9556. doi: 10.1126/scitranslmed.adf9556. Epub 2023 Oct 4. Sci Transl Med. 2023. PMID: 37792959 Free PMC article.
-
Divergent EGFR/MAPK-Mediated Immune Responses to Clinical Candida Pathogens in Vulvovaginal Candidiasis.Front Immunol. 2022 May 26;13:894069. doi: 10.3389/fimmu.2022.894069. eCollection 2022. Front Immunol. 2022. PMID: 35720274 Free PMC article.
-
Profiling inflammatory outcomes of Candida albicans colonization and food allergy induction in the murine glandular stomach.mBio. 2024 Nov 13;15(11):e0211324. doi: 10.1128/mbio.02113-24. Epub 2024 Sep 30. mBio. 2024. PMID: 39347572 Free PMC article.
-
The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: current knowledge and new perspectives.FEMS Microbiol Rev. 2021 May 5;45(3):fuaa060. doi: 10.1093/femsre/fuaa060. FEMS Microbiol Rev. 2021. PMID: 33232448 Free PMC article. Review.
References
-
- Calderone R.A., editor. Candida and Candidiasis. ASM Press; Washington, DC, USA: 2012.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

