Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma
- PMID: 32219428
- PMCID: PMC7101507
- DOI: 10.1001/jama.2020.4783
Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma
Abstract
Importance: Coronavirus disease 2019 (COVID-19) is a pandemic with no specific therapeutic agents and substantial mortality. It is critical to find new treatments.
Objective: To determine whether convalescent plasma transfusion may be beneficial in the treatment of critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Design, setting, and participants: Case series of 5 critically ill patients with laboratory-confirmed COVID-19 and acute respiratory distress syndrome (ARDS) who met the following criteria: severe pneumonia with rapid progression and continuously high viral load despite antiviral treatment; Pao2/Fio2 <300; and mechanical ventilation. All 5 were treated with convalescent plasma transfusion. The study was conducted at the infectious disease department, Shenzhen Third People's Hospital in Shenzhen, China, from January 20, 2020, to March 25, 2020; final date of follow-up was March 25, 2020. Clinical outcomes were compared before and after convalescent plasma transfusion.
Exposures: Patients received transfusion with convalescent plasma with a SARS-CoV-2-specific antibody (IgG) binding titer greater than 1:1000 (end point dilution titer, by enzyme-linked immunosorbent assay [ELISA]) and a neutralization titer greater than 40 (end point dilution titer) that had been obtained from 5 patients who recovered from COVID-19. Convalescent plasma was administered between 10 and 22 days after admission.
Main outcomes and measures: Changes of body temperature, Sequential Organ Failure Assessment (SOFA) score (range 0-24, with higher scores indicating more severe illness), Pao2/Fio2, viral load, serum antibody titer, routine blood biochemical index, ARDS, and ventilatory and extracorporeal membrane oxygenation (ECMO) supports before and after convalescent plasma transfusion.
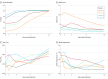
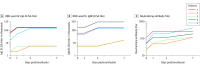
Results: All 5 patients (age range, 36-65 years; 2 women) were receiving mechanical ventilation at the time of treatment and all had received antiviral agents and methylprednisolone. Following plasma transfusion, body temperature normalized within 3 days in 4 of 5 patients, the SOFA score decreased, and Pao2/Fio2 increased within 12 days (range, 172-276 before and 284-366 after). Viral loads also decreased and became negative within 12 days after the transfusion, and SARS-CoV-2-specific ELISA and neutralizing antibody titers increased following the transfusion (range, 40-60 before and 80-320 on day 7). ARDS resolved in 4 patients at 12 days after transfusion, and 3 patients were weaned from mechanical ventilation within 2 weeks of treatment. Of the 5 patients, 3 have been discharged from the hospital (length of stay: 53, 51, and 55 days), and 2 are in stable condition at 37 days after transfusion.
Conclusions and relevance: In this preliminary uncontrolled case series of 5 critically ill patients with COVID-19 and ARDS, administration of convalescent plasma containing neutralizing antibody was followed by improvement in their clinical status. The limited sample size and study design preclude a definitive statement about the potential effectiveness of this treatment, and these observations require evaluation in clinical trials.
Conflict of interest statement
Figures
Comment in
-
Convalescent Plasma to Treat COVID-19: Possibilities and Challenges.JAMA. 2020 Apr 28;323(16):1561-1562. doi: 10.1001/jama.2020.4940. JAMA. 2020. PMID: 32219429 No abstract available.
-
COVID-19: are neutralizing antibodies neutralizing enough?Transfusion. 2020 Jul;60(7):1602-1603. doi: 10.1111/trf.15897. Epub 2020 Jun 3. Transfusion. 2020. PMID: 32449171 Free PMC article. No abstract available.
-
Compassionate use of others' immunity - understanding gut microbiome in Covid-19.Crit Care. 2020 Jun 18;24(1):358. doi: 10.1186/s13054-020-03043-w. Crit Care. 2020. PMID: 32552848 Free PMC article. No abstract available.
Similar articles
-
Evaluating the efficacy and safety of human anti-SARS-CoV-2 convalescent plasma in severely ill adults with COVID-19: A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 8;21(1):499. doi: 10.1186/s13063-020-04422-y. Trials. 2020. PMID: 32513308 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2 PMID: 32406927 Free PMC article. Updated.
-
Convalescent plasma transfusion therapy in severe COVID-19 patients- a safety, efficacy and dose response study: A structured summary of a study protocol of a phase II randomized controlled trial.Trials. 2020 Oct 26;21(1):883. doi: 10.1186/s13063-020-04734-z. Trials. 2020. PMID: 33106167 Free PMC article. Clinical Trial.
-
Convalescent plasma - Is it useful for treating SARS Co-V2 infection?Indian J Med Microbiol. 2020 Jul-Dec;38(3 & 4):252-260. doi: 10.4103/ijmm.IJMM_20_358. Indian J Med Microbiol. 2020. PMID: 33154232 Free PMC article. Review.
-
Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks.Clin Microbiol Infect. 2020 Oct;26(10):1436-1446. doi: 10.1016/j.cmi.2020.08.005. Epub 2020 Aug 11. Clin Microbiol Infect. 2020. PMID: 32791241 Free PMC article. Review.
Cited by
-
Cerebellar infarction requiring surgical decompression in patient with COVID 19 pathological analysis and brief review.Interdiscip Neurosurg. 2020 Dec;22:100850. doi: 10.1016/j.inat.2020.100850. Epub 2020 Jul 29. Interdiscip Neurosurg. 2020. PMID: 32835021 Free PMC article.
-
Current Status of COVID-19 Therapies and Drug Repositioning Applications.iScience. 2020 Jul 24;23(7):101303. doi: 10.1016/j.isci.2020.101303. Epub 2020 Jun 20. iScience. 2020. PMID: 32622261 Free PMC article. Review.
-
SARS-CoV-2 Antibody Avidity Responses in COVID-19 Patients and Convalescent Plasma Donors.J Infect Dis. 2020 Nov 13;222(12):1974-1984. doi: 10.1093/infdis/jiaa581. J Infect Dis. 2020. PMID: 32910175 Free PMC article.
-
A comprehensive, longitudinal analysis of humoral responses specific to four recombinant antigens of SARS-CoV-2 in severe and non-severe COVID-19 patients.PLoS Pathog. 2020 Sep 10;16(9):e1008796. doi: 10.1371/journal.ppat.1008796. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32913364 Free PMC article.
-
Differential persistence of neutralizing antibody against SARS-CoV-2 in post immunized Bangladeshi population.Sci Rep. 2022 Aug 29;12(1):14681. doi: 10.1038/s41598-022-18302-9. Sci Rep. 2022. PMID: 36038600 Free PMC article.
References
-
- WHO Novel coronavirus (COVID-19) situation. Updated March 24, 2020. https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. Published online February 24, 2020. doi:10.1001/jama.2020.2648 - DOI - PubMed
-
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;14(1):69-71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

