Bromodomains and Extra-Terminal (BET) Inhibitor JQ1 Suppresses Proliferation of Acute Lymphocytic Leukemia by Inhibiting c-Myc-Mediated Glycolysis
- PMID: 32266878
- PMCID: PMC7165247
- DOI: 10.12659/MSM.923411
Bromodomains and Extra-Terminal (BET) Inhibitor JQ1 Suppresses Proliferation of Acute Lymphocytic Leukemia by Inhibiting c-Myc-Mediated Glycolysis
Abstract
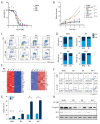
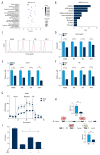
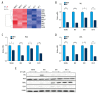
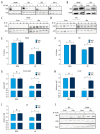
BACKGROUND Acute lymphocytic leukemia (ALL) is a common blood cancer which induces high mortality in children. Bromodomains and extra-terminal (BET) protein inhibitors, such as JQ1 and ARV-825, are promising cancer therapeutic agents that can be used by _targeting c-Myc. A recent work reported that JQ1 effectively attenuates ALL in vitro by suppressing cell proliferation and accelerating apoptosis. The purpose of this research was to probe into the potential mechanism of how JQ1 inhibits ALL cell proliferation in vitro. MATERIAL AND METHODS Cell viability of ALL cells were measured by CTG after treatment by JQ1. Cell cycle analysis was done by EdU and PI staining. Cell apoptosis was assessed by Annexin V/PI staining. Glycolysis was detected using Seahorse and LC-MS kits. The expression of glycolytic rate-limiting enzymes was assessed by RNA-seq, qRT-PCR, and Western blot. RESULTS JQ1 suppressed cell proliferation by arresting the cell cycle and inducing the apoptosis of acute lymphocytic leukemia cells. JQ1 inhibited cell proliferation of B-ALL cells by restraining glycolysis. Conversely, the cell cycle block of B-ALL cells induced by JQ1 was partially abolished after pretreatment with 2-Deoxy-D-glucose (2-DG), an inhibitor of glycolysis. Furthermore, JQ1 restrained the glycolysis of B-ALL cell lines by remarkably downregulating the rate-limiting enzymes of glycolysis, such as hexokinase 2, phosphofructokinase, and lactate dehydrogenase A. Moreover, the cell cycle arrest was reversed in B-ALL cells with overexpressed c-Myc treated by JQ1, which is involved in the enhancement of glycolysis. CONCLUSIONS The BET inhibitor JQ1 suppresses the proliferation of ALL by inhibiting c-Myc-mediated glycolysis, thus providing a new strategy for the treatment of ALL.
Conflict of interest statement
None.
Figures
Similar articles
-
The BET Bromodomain Inhibitor JQ1 Suppresses Chondrosarcoma Cell Growth via Regulation of YAP/p21/c-Myc Signaling.J Cell Biochem. 2017 Aug;118(8):2182-2192. doi: 10.1002/jcb.25863. Epub 2017 Apr 18. J Cell Biochem. 2017. PMID: 28059436
-
BET bromodomain inhibition _targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia.Blood. 2012 Oct 4;120(14):2843-52. doi: 10.1182/blood-2012-02-413021. Epub 2012 Aug 17. Blood. 2012. PMID: 22904298 Free PMC article.
-
Pharmacological _targeting of BET Bromodomains Inhibits Lens Fibrosis via Downregulation of MYC Expression.Invest Ophthalmol Vis Sci. 2019 Nov 1;60(14):4748-4758. doi: 10.1167/iovs.19-27596. Invest Ophthalmol Vis Sci. 2019. PMID: 31731295
-
JQ1: a novel potential therapeutic _target.Pharmazie. 2018 Sep 1;73(9):491-493. doi: 10.1691/ph.2018.8480. Pharmazie. 2018. PMID: 30223929 Review.
-
Progress in the development of domain selective inhibitors of the bromo and extra terminal domain family (BET) proteins.Eur J Med Chem. 2021 Dec 15;226:113853. doi: 10.1016/j.ejmech.2021.113853. Epub 2021 Sep 13. Eur J Med Chem. 2021. PMID: 34547507 Review.
Cited by
-
Deregulated transcription factors in cancer cell metabolisms and reprogramming.Semin Cancer Biol. 2022 Nov;86(Pt 3):1158-1174. doi: 10.1016/j.semcancer.2022.10.001. Epub 2022 Oct 13. Semin Cancer Biol. 2022. PMID: 36244530 Free PMC article. Review.
-
Mechanisms of Immunosuppressive Tumor Evasion: Focus on Acute Lymphoblastic Leukemia.Front Immunol. 2021 Nov 18;12:737340. doi: 10.3389/fimmu.2021.737340. eCollection 2021. Front Immunol. 2021. PMID: 34867958 Free PMC article. Review.
-
Metabolic Reprogramming by Ribitol Expands the Therapeutic Window of BETi JQ1 against Breast Cancer.Cancers (Basel). 2023 Sep 1;15(17):4356. doi: 10.3390/cancers15174356. Cancers (Basel). 2023. PMID: 37686632 Free PMC article.
-
Co-overexpression of BRD4 and CDK7 promotes cell proliferation and predicts poor prognosis in HCC.Heliyon. 2024 Jan 9;10(2):e24389. doi: 10.1016/j.heliyon.2024.e24389. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38293462 Free PMC article.
-
Lactate Utilization Enables Metabolic Escape to Confer Resistance to BET Inhibition in Acute Myeloid Leukemia.Cancer Res. 2024 Apr 1;84(7):1101-1114. doi: 10.1158/0008-5472.CAN-23-0291. Cancer Res. 2024. PMID: 38285895 Free PMC article.
References
-
- Wu C, Li W. Genomics and pharmacogenomics of pediatric acute lymphoblastic leukemia. Crit Rev Oncol Hematol. 2018;126:100–11. - PubMed
-
- Uderzo C, Dini G, Locatelli F, et al. Treatment of childhood acute lymphoblastic leukemia after the first relapse: Curative strategies. Haematologica. 2000;85:47–53. - PubMed
-
- Kamel-Reid S, Letarte M, Doedens M, et al. Bone marrow from children in relapse with pre-B acute lymphoblastic leukemia proliferates and disseminates rapidly in scid mice. Blood. 1991;78:2973–81. - PubMed
-
- Bose S, Le A. Glucose metabolism in cancer. Adv Exp Med Biol. 2018;1063:3–12. - PubMed
-
- Vaupel P, Schmidberger H, Mayer A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

