Porcine deltacoronavirus activates the Raf/MEK/ERK pathway to promote its replication
- PMID: 32283129
- PMCID: PMC7194644
- DOI: 10.1016/j.virusres.2020.197961
Porcine deltacoronavirus activates the Raf/MEK/ERK pathway to promote its replication
Abstract
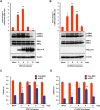
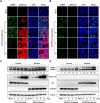
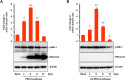
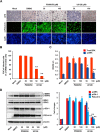
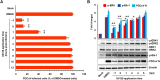
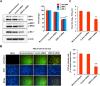
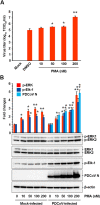
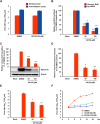
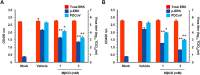
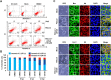
Porcine deltacoronavirus (PDCoV) is a newly emerged swine coronavirus that causes acute enteritis in neonatal piglets. To date, little is known about the host factors or cellular signaling mechanisms associated with PDCoV replication. Since the Raf/MEK/ERK pathway is involved in modulation of various important cellular functions, numerous DNA and RNA viruses coopt this pathway for efficient propagation. In the present study, we found that PDCoV induces the activation of ERK1/2 and its downstream substrate Elk-1 early in infection irrespective of viral biosynthesis. Chemical inhibition or knockdown of ERK1/2 significantly suppressed viral replication, whereas treatment with an ERK activator increased viral yields. Direct pharmacological inhibition of ERK activation had no effect on the viral entry process but sequentially affected the post-entry steps of the virus life cycle. In addition, pharmacological sequestration of cellular or viral cholesterol downregulated PDCoV-induced ERK signaling, highlighting the significance of the cholesterol contents in ERK activation. However, ERK inhibition had no effect on PDCoV-triggered apoptosis through activation of the cytochrome c-mediated intrinsic mitochondrial pathway, suggesting the irrelevance of ERK activation to the apoptosis pathway during PDCoV infection. Altogether, our findings indicate that the ERK signaling pathway plays a pivotal role in viral biosynthesis to facilitate the optimal replication of PDCoV.
Keywords: Cholesterol; ERK1/2; PDCoV; Signal transduction; Viral replication.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no conflict of interest.
Figures
Similar articles
-
NLRP1 restricts porcine deltacoronavirus infection via IL-11 inhibiting the phosphorylation of the ERK signaling pathway.J Virol. 2024 Mar 19;98(3):e0198223. doi: 10.1128/jvi.01982-23. Epub 2024 Feb 27. J Virol. 2024. PMID: 38411106 Free PMC article.
-
Porcine deltacoronavirus nucleocapsid protein interacts with the Grb2 through its proline-rich motifs to induce activation of the Raf-MEK-ERK signal pathway and promote virus replication.J Gen Virol. 2024 Aug;105(8). doi: 10.1099/jgv.0.002014. J Gen Virol. 2024. PMID: 39136113
-
Porcine deltacoronavirus induces caspase-dependent apoptosis through activation of the cytochrome c-mediated intrinsic mitochondrial pathway.Virus Res. 2018 Jul 15;253:112-123. doi: 10.1016/j.virusres.2018.06.008. Epub 2018 Jun 22. Virus Res. 2018. PMID: 29940190 Free PMC article.
-
Porcine deltacoronavirus infection: Etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis.Virus Res. 2016 Dec 2;226:50-59. doi: 10.1016/j.virusres.2016.04.009. Epub 2016 Apr 13. Virus Res. 2016. PMID: 27086031 Free PMC article. Review.
-
ERK1/2 MAP kinases: structure, function, and regulation.Pharmacol Res. 2012 Aug;66(2):105-43. doi: 10.1016/j.phrs.2012.04.005. Epub 2012 Apr 27. Pharmacol Res. 2012. PMID: 22569528 Review.
Cited by
-
Repurposing Anticancer Drugs _targeting the MAPK/ERK Signaling Pathway for the Treatment of Respiratory Virus Infections.Int J Mol Sci. 2024 Jun 25;25(13):6946. doi: 10.3390/ijms25136946. Int J Mol Sci. 2024. PMID: 39000055 Free PMC article. Review.
-
Nicotinamide Efficiently Suppresses Porcine Epidemic Diarrhea Virus and Porcine Deltacoronavirus Replication.Viruses. 2023 Jul 21;15(7):1591. doi: 10.3390/v15071591. Viruses. 2023. PMID: 37515276 Free PMC article.
-
The MEK1/2-inhibitor ATR-002 efficiently blocks SARS-CoV-2 propagation and alleviates pro-inflammatory cytokine/chemokine responses.Cell Mol Life Sci. 2022 Jan 10;79(1):65. doi: 10.1007/s00018-021-04085-1. Cell Mol Life Sci. 2022. PMID: 35013790 Free PMC article.
-
Swine acute diarrhea syndrome coronavirus replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway.Virology. 2022 Jan 2;565:96-105. doi: 10.1016/j.virol.2021.10.009. Epub 2021 Oct 30. Virology. 2022. PMID: 34768113 Free PMC article.
-
NLRP1 restricts porcine deltacoronavirus infection via IL-11 inhibiting the phosphorylation of the ERK signaling pathway.J Virol. 2024 Mar 19;98(3):e0198223. doi: 10.1128/jvi.01982-23. Epub 2024 Feb 27. J Virol. 2024. PMID: 38411106 Free PMC article.
References
-
- Abkhezr M., Keramati A.R., Ostad S.N., Davoodi J., Ghahremani M.H. The time course of Akt and ERK activation on XIAP expression in HEK 293 cell line. Mol. Biol. Rep. 2010;37:2037–2042. - PubMed
-
- Alessi D.R., Cuenda A., Cohen P., Dudley D.T., Saltiel A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. - PubMed
-
- Cagnol S., Chambard J.C. ERK and cell death: mechanisms of ERK-induced cell death-apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

