The Synergistic Anti-Cancer Effects of NVP-BEZ235 and Regorafenib in Hepatocellular Carcinoma
- PMID: 32466169
- PMCID: PMC7287658
- DOI: 10.3390/molecules25102454
The Synergistic Anti-Cancer Effects of NVP-BEZ235 and Regorafenib in Hepatocellular Carcinoma
Abstract
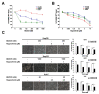
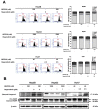
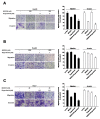
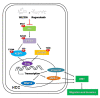
Hepatocellular carcinoma (HCC) is the most common type of liver cancer worldwide. Regorafenib is a multi-kinase inhibitor and the second-line treatment for HCC. Since the PI3K/Akt/mTOR signaling pathway is dysregulated in HCC, we evaluated the therapeutic effects of regorafenib combined with a dual PI3K/mTOR inhibitor BEZ235 in the human HCC cell lines (n = 3). The combined treatment with BEZ235 and regorafenib enhanced the inhibition of cell proliferation and increased the expression of cleaved caspase-3 and cleaved PARP in HCC cells. Moreover, the combined treatment suppressed HCC cell migration and invasion in the transwell assay. Further, the Western blot analyses confirmed the involvement of epithelial-mesenchymal transition (EMT)-related genes such as slug, vimentin, and matrix metalloproteinase (MMP)-9/-2. Additionally, the proteinase activity of MMP-9/-2 was analyzed using gelatin zymography. Furthermore, the inhibition of phosphorylation of the Akt, mTOR, p70S6K, and 4EBP1 after combined treatment was validated using Western blot analysis. Therefore, these results suggest that the combined treatment with BEZ235 and regorafenib benefits patients with HCC.
Keywords: BEZ235; PI3K/Akt/mTOR pathway; combination therapy; hepatocellular carcinoma; metastasis; regorafenib.
Conflict of interest statement
The authors declare no conflict interests.
Figures


Similar articles
-
[β-Lapachone combined with NVP-BEZ235 inhibit proliferation and migration of BGC-823 gastric cancer cells].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018 Feb;34(2):129-135. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018. PMID: 29673455 Chinese.
-
Inhibition of autophagy enhances apoptosis induced by the PI3K/AKT/mTor inhibitor NVP-BEZ235 in renal cell carcinoma cells.Cell Biochem Funct. 2013 Jul;31(5):427-33. doi: 10.1002/cbf.2917. Epub 2012 Oct 22. Cell Biochem Funct. 2013. PMID: 23086777
-
NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells.Cancer Lett. 2015 Oct 10;367(1):58-68. doi: 10.1016/j.canlet.2015.07.007. Epub 2015 Jul 15. Cancer Lett. 2015. PMID: 26188279
-
Role of cellular, molecular and tumor microenvironment in hepatocellular carcinoma: Possible _targets and future directions in the regorafenib era.Int J Cancer. 2020 Oct 1;147(7):1778-1792. doi: 10.1002/ijc.32970. Epub 2020 Apr 15. Int J Cancer. 2020. PMID: 32162677 Review.
-
Akt scaffold proteins: the key to controlling specificity of Akt signaling.Am J Physiol Cell Physiol. 2021 Sep 1;321(3):C429-C442. doi: 10.1152/ajpcell.00146.2020. Epub 2021 Jun 23. Am J Physiol Cell Physiol. 2021. PMID: 34161152 Review.
Cited by
-
The essential roles of lncRNAs/PI3K/AKT axis in gastrointestinal tumors.Front Cell Dev Biol. 2024 Aug 5;12:1442193. doi: 10.3389/fcell.2024.1442193. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39161590 Free PMC article. Review.
-
A feedback loop of PPP and PI3K/AKT signal pathway drives regorafenib-resistance in HCC.Cancer Metab. 2023 Dec 18;11(1):27. doi: 10.1186/s40170-023-00311-5. Cancer Metab. 2023. PMID: 38111012 Free PMC article.
-
Regorafenib and Ruthenium Complex Combination Inhibit Cancer Cell Growth by _targeting PI3K/AKT/ERK Signalling in Colorectal Cancer Cells.Int J Mol Sci. 2022 Dec 30;24(1):686. doi: 10.3390/ijms24010686. Int J Mol Sci. 2022. PMID: 36614133 Free PMC article.
-
TOP2A inhibition reverses drug resistance of hepatocellular carcinoma to regorafenib.Am J Cancer Res. 2022 Sep 15;12(9):4343-4360. eCollection 2022. Am J Cancer Res. 2022. PMID: 36225636 Free PMC article.
-
The interactions between integrin α5β1 of liver cancer cells and fibronectin of fibroblasts promote tumor growth and angiogenesis.Int J Biol Sci. 2022 Aug 1;18(13):5019-5037. doi: 10.7150/ijbs.72367. eCollection 2022. Int J Biol Sci. 2022. PMID: 35982891 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

