The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease
- PMID: 32485838
- PMCID: PMC7312931
- DOI: 10.3390/ijms21113863
The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease
Abstract
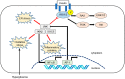
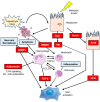
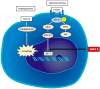
Nonalcoholic fatty liver disease (NAFLD) consists of the entire spectrum of fatty liver disease in patients without significant alcohol consumption, ranging from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) to cirrhosis, with NASH recently shown as an important cause of hepatocellular carcinoma (HCC). There is a close relationship between insulin resistance (IR) and NAFLD, with a five-fold higher prevalence of NAFLD in patients with type 2 diabetes (T2DM) compared to that in patients without T2DM. IR is involved in the progression of disease conditions such as steatosis and NASH, as well as hepatic fibrosis progression. The mechanisms underlying these processes involve genetic factors, hepatic fat accumulation, alterations in energy metabolism, and inflammatory signals derived from various cell types including immune cells. In NASH-associated fibrosis, the principal cell type responsible for extracellular matrix production is the hepatic stellate cell (HSC). HSC activation by IR involves "direct" and "indirect" pathways. This review will describe the molecular mechanisms of inflammation and hepatic fibrosis in IR, the relationship between T2DM and hepatic fibrosis, and the relationship between T2DM and HCC in patients with NAFLD.
Keywords: hepatic fibrosis; hepatocellular carcinoma; inflammation; insulin resistance; stellate cell.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Molecular Mechanisms of Nonalcoholic Fatty Liver Disease (NAFLD)/Nonalcoholic Steatohepatitis (NASH).Adv Exp Med Biol. 2021;1261:223-229. doi: 10.1007/978-981-15-7360-6_20. Adv Exp Med Biol. 2021. PMID: 33783745
-
Prevalence of Nonalcoholic Steatohepatitis-Associated Cirrhosis in the United States: An Analysis of National Health and Nutrition Examination Survey Data.Am J Gastroenterol. 2017 Apr;112(4):581-587. doi: 10.1038/ajg.2017.5. Epub 2017 Feb 14. Am J Gastroenterol. 2017. PMID: 28195177
-
IMPACT OF TYPE 2 DIABETES ON NONALCOHOLIC STEATOHEPATITIS AND ADVANCED FIBROSIS IN PATIENTS WITH NONALCOHOLIC FATTY LIVER DISEASE.Endocr Pract. 2020 Apr;26(4):444-453. doi: 10.4158/EP-2019-0342. Epub 2020 Jan 22. Endocr Pract. 2020. PMID: 31968197
-
Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma.Int J Mol Sci. 2020 Feb 23;21(4):1525. doi: 10.3390/ijms21041525. Int J Mol Sci. 2020. PMID: 32102237 Free PMC article. Review.
-
Non-alcoholic fatty liver disease and type 2 diabetes mellitus: the liver disease of our age?World J Gastroenterol. 2014 Jul 21;20(27):9072-89. doi: 10.3748/wjg.v20.i27.9072. World J Gastroenterol. 2014. PMID: 25083080 Free PMC article. Review.
Cited by
-
Biological Role and Related Natural Products of SIRT1 in Nonalcoholic Fatty Liver.Diabetes Metab Syndr Obes. 2023 Dec 8;16:4043-4064. doi: 10.2147/DMSO.S437865. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 38089432 Free PMC article. Review.
-
The triglyceride and glucose index and risk of nonalcoholic fatty liver disease: A dose-response meta-analysis.Front Endocrinol (Lausanne). 2023 Jan 19;13:1043169. doi: 10.3389/fendo.2022.1043169. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36743937 Free PMC article.
-
Lipotoxicity as the Leading Cause of Non-Alcoholic Steatohepatitis.Int J Mol Sci. 2022 May 5;23(9):5146. doi: 10.3390/ijms23095146. Int J Mol Sci. 2022. PMID: 35563534 Free PMC article. Review.
-
Advances in the Pathogenesis of Metabolic Liver Disease-Related Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2024 Mar 19;11:581-594. doi: 10.2147/JHC.S450460. eCollection 2024. J Hepatocell Carcinoma. 2024. PMID: 38525158 Free PMC article. Review.
-
Gender Differences in the Relationships among Metabolic Syndrome and Various Obesity-Related Indices with Nonalcoholic Fatty Liver Disease in a Taiwanese Population.Int J Environ Res Public Health. 2021 Jan 20;18(3):857. doi: 10.3390/ijerph18030857. Int J Environ Res Public Health. 2021. PMID: 33498329 Free PMC article.
References
-
- Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. - DOI - PubMed
-
- European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. - DOI - PubMed
-
- Wong V.W., Chan W.K., Chitturi S., Chawla Y., Dan Y.Y., Duseja A., Fan J., Goh K.L., Hamaguchi M., Hashimoto E., et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: Definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

