Effect of Rubusoside, a Natural Sucrose Substitute, on Streptococcus mutans Biofilm Cariogenic Potential and Virulence Gene Expression In Vitro
- PMID: 32503907
- PMCID: PMC7414950
- DOI: 10.1128/AEM.01012-20
Effect of Rubusoside, a Natural Sucrose Substitute, on Streptococcus mutans Biofilm Cariogenic Potential and Virulence Gene Expression In Vitro
Abstract
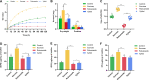
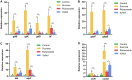
Dental caries is a biofilm-mediated disease in which Streptococcus mutans is the main pathogenic microorganism, and its incidence is closely related to sucrose. Rubusoside is a natural nonnutritive sweetener isolated from Rubus suavissimus S. Lee. This study was designed to determine the effect of this sucrose substitute on the cariogenic properties and virulence gene expression of S. mutans biofilms. S. mutans was exposed to brain heart infusion (BHI) medium (as a control), 1% sucrose-supplemented medium, 1% rubusoside-supplemented medium, and 1% xylitol-supplemented medium. The growth curve of the biofilm was monitored by crystal violet staining, and the pH was measured every 24 h. After 5 days, the biofilms formed on the glass coverslips were recovered to determine the biomass (dry weight and total amount of soluble proteins), numbers of CFU, and amounts of intra- and extracellular polysaccharides. Biofilm structural imaging was performed using a scanning electron microscope (SEM). Virulence gene expression (gtfB, gtfC, gtfD, ftf, spaP, gbpB, ldh, atpF, vicR, and comD) was determined by reverse transcription-quantitative PCR. Growth in rubusoside resulted in lower levels of acid production than observed during growth in sucrose, xylitol, and the control, while it also reduced the level of biofilm accumulation and bacterial viability and even reduced the level of production of extracellular polysaccharides. By SEM, the levels of biofilm formation and extracellular matrix during growth in rubusoside were lower than these levels during growth in sucrose and xylitol. From the perspective of virulence genes, growth in rubusoside and xylitol significantly inhibited the expression of virulence genes compared with their levels of expression after growth in sucrose. Among these genes, gtfB, gtfC, gbpB, ldh, and comD downregulation was found with growth in rubusoside compared with their expression with growth in xylitol. Therefore, rubusoside appears to be less potentially cariogenic than sucrose and xylitol and may become an effective sucrose substitute for caries prevention. Further studies are needed to deepen these findings.IMPORTANCE Dental caries is a major public health challenge and places heavy biological, social, and financial burdens on individuals and health care systems. To palliate the deleterious effect of sucrose on the virulence factors of S. mutans, massive commercial efforts have been oriented toward developing products that may act as sucrose substitutes. Rubusoside, a natural sucrose substitute, is a plant extract with a high level of sweetness. Although some studies have shown that rubusoside does not produce acids or inhibit the growth of S. mutans, little attention has been paid to its effect on dental biofilm and the underlying mechanisms. Our study focuses on the effect of rubusoside on the formation and structure of biofilms and the expression of virulence genes. The results confirm that rubusoside can inhibit accumulation, bacterial viability, polysaccharide production by the biofilm, and related gene expression. These results provide further insight into the cariogenicity of S. mutans biofilms and demonstrate a new perspective for studying the impact of sucrose substitutes on caries.
Keywords: Streptococcus mutans; biofilm formation; dental caries; rubusoside; virulence gene.
Copyright © 2020 American Society for Microbiology.
Figures

Similar articles
-
Cariogenicity features of Streptococcus mutans in presence of rubusoside.BMC Oral Health. 2016 May 11;16(1):54. doi: 10.1186/s12903-016-0212-1. BMC Oral Health. 2016. PMID: 27169524 Free PMC article.
-
Sucrose substitutes affect the cariogenic potential of Streptococcus mutans biofilms.Caries Res. 2014;48(3):214-22. doi: 10.1159/000354410. Epub 2014 Jan 29. Caries Res. 2014. PMID: 24481032
-
Utilization of the extract of Cedrus deodara (Roxb. ex D.Don) G. Don against the biofilm formation and the expression of virulence genes of cariogenic bacterium Streptococcus mutans.J Ethnopharmacol. 2020 Jul 15;257:112856. doi: 10.1016/j.jep.2020.112856. Epub 2020 Apr 9. J Ethnopharmacol. 2020. PMID: 32278760
-
Inhibition of Streptococcus mutans biofilm formation by strategies _targeting the metabolism of exopolysaccharides.Crit Rev Microbiol. 2021 Sep;47(5):667-677. doi: 10.1080/1040841X.2021.1915959. Epub 2021 May 3. Crit Rev Microbiol. 2021. PMID: 33938347 Review.
-
Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms.Caries Res. 2011;45(1):69-86. doi: 10.1159/000324598. Epub 2011 Feb 23. Caries Res. 2011. PMID: 21346355 Free PMC article. Review.
Cited by
-
Streptococcus mutans suppresses filamentous growth of Candida albicans through secreting mutanocyclin, an unacylated tetramic acid.Virulence. 2022 Dec;13(1):542-557. doi: 10.1080/21505594.2022.2046952. Virulence. 2022. PMID: 35311622 Free PMC article.
-
Lactobacillus cell envelope-coated nanoparticles for antibiotic delivery against cariogenic biofilm and dental caries.J Nanobiotechnology. 2022 Aug 2;20(1):356. doi: 10.1186/s12951-022-01563-x. J Nanobiotechnology. 2022. PMID: 35918726 Free PMC article.
-
Alteration of oral microbial biofilms by sweeteners.Biofilm. 2023 Dec 13;7:100171. doi: 10.1016/j.bioflm.2023.100171. eCollection 2024 Jun. Biofilm. 2023. PMID: 38197082 Free PMC article. Review.
-
Silica nanoparticles containing nano-silver and chlorhexidine to suppress Porphyromonas gingivalis biofilm and modulate multispecies biofilms toward healthy tendency.J Oral Microbiol. 2024 Jun 5;16(1):2361403. doi: 10.1080/20002297.2024.2361403. eCollection 2024. J Oral Microbiol. 2024. PMID: 38847000 Free PMC article.
-
Unveiling the complexity of early childhood caries: Candida albicans and Streptococcus mutans cooperative strategies in carbohydrate metabolism and virulence.J Oral Microbiol. 2024 Apr 10;16(1):2339161. doi: 10.1080/20002297.2024.2339161. eCollection 2024. J Oral Microbiol. 2024. PMID: 38606339 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

