Adaptive expression of biofilm regulators and adhesion factors of Staphylococcus aureus during acute wound infection under the treatment of negative pressure wound therapy in vivo
- PMID: 32509022
- PMCID: PMC7271737
- DOI: 10.3892/etm.2020.8679
Adaptive expression of biofilm regulators and adhesion factors of Staphylococcus aureus during acute wound infection under the treatment of negative pressure wound therapy in vivo
Abstract
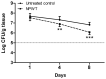
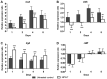
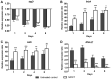
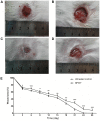
Negative pressure wound therapy (NPWT) is gaining acceptance as a physical therapy for a wide variety of infected wounds. To gain insight into the response of bacteria to NPWT in vivo, the adaptive expression of biofilm regulators and adhesion factors of Staphylococcus aureus (S. aureus), the most frequently isolated pathogen in the clinic, during acute wound infection was investigated. A 3 cm full-thickness dermal wound was created on each side of a rabbit back and inoculated with green fluorescent protein-labeled S. aureus. NPWT was initiated at 6 h post inoculation, with the wound on the contralateral side as the untreated self-control. The wounds were subjected to a 28 day observation period. Histological analysis, laser scanning confocal microscopy and scanning electron microscopy revealed a transition of S. aureus to a free-living phenotype in tissues treated with NPWT, compared with microcolonies in untreated wounds. Viable bacteria counts showed a modest reduction in the bioburden of NPWT group on day 8 (P<0.001), with ~1x106 colony-forming units/g tissue. Transcript analysis of biofilm- and colonization-related genes were investigated using reverse transcription-quantitative PCR on postoperative days 1, 2, 4 and 8. The poly-beta-1,6-N-acetyl-D-glucosamine synthase locus and holin-like protein CidA/antiholin-like protein LrgA network were less active in the NPWT group compared with the untreated control group. Accordingly, the expression profile switched to an elevated expression of the adhesive factors UDP-phosphate N-acetylglucosaminyl 1-phosphate transferase (at days 0-4) and fibronectin-binding protein A and iron-regulated surface determinant protein A at >4 days during both stages of colonization. Meanwhile, low expression levels of the effector molecule (RNAIII) of the accessory gene regulator type I (agr) system was detected in NPWT group, suggesting that the bacterial density in NPWT-treated wounds was under the threshold for agr activation, thus not leading to an active and invasive infection. The wounds treated by NPWT healed completely on day 28, compared with an average of an 8.11% defect area in the control group (P<0.001). The results of the current study indicated that S. aureus responds to NPWT by regulating gene expression, manifesting a decrease in biofilm formation and an increase in bacterial colonization in vivo, which potentially benefits the wound repair and healing process.
Keywords: Staphylococcus aureus; acute wound; bacterial colonization; biofilm; negative pressure wound therapy.
Copyright: © Li et al.
Figures

Similar articles
-
Early application of negative pressure wound therapy to acute wounds contaminated with Staphylococcus aureus: An effective approach to preventing biofilm formation.Exp Ther Med. 2016 Mar;11(3):769-776. doi: 10.3892/etm.2016.3008. Epub 2016 Jan 20. Exp Ther Med. 2016. PMID: 26997991 Free PMC article.
-
Impact of negative-pressure wound therapy on bacterial behaviour and bioburden in a contaminated full-thickness wound.Int Wound J. 2019 Oct;16(5):1214-1221. doi: 10.1111/iwj.13197. Epub 2019 Sep 4. Int Wound J. 2019. PMID: 31483575 Free PMC article.
-
Negative-pressure wound therapy enhances local inflammatory responses in acute infected soft-tissue wound.Cell Biochem Biophys. 2014 Sep;70(1):539-47. doi: 10.1007/s12013-014-9953-0. Cell Biochem Biophys. 2014. PMID: 24748178
-
Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention.Cochrane Database Syst Rev. 2012 Apr 18;(4):CD009261. doi: 10.1002/14651858.CD009261.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Oct 07;(10):CD009261. doi: 10.1002/14651858.CD009261.pub3 PMID: 22513974 Updated. Review.
-
Use of negative-pressure wound therapy in orthopaedic trauma.J Am Acad Orthop Surg. 2012 Sep;20(9):564-74. doi: 10.5435/JAAOS-20-09-564. J Am Acad Orthop Surg. 2012. PMID: 22941799 Review.
Cited by
-
Comparison of the effects of negative pressure wound therapy and negative pressure wound therapy with instillation on wound healing in a porcine model.Front Surg. 2023 Apr 17;10:1080838. doi: 10.3389/fsurg.2023.1080838. eCollection 2023. Front Surg. 2023. PMID: 37139193 Free PMC article.
-
Clinical outcomes of negative pressure wound therapy with instillation vs standard negative pressure wound therapy for wounds: A meta-analysis of randomised controlled trials.Int Wound J. 2023 May;20(5):1739-1749. doi: 10.1111/iwj.13989. Epub 2022 Dec 15. Int Wound J. 2023. PMID: 36519410 Free PMC article.
-
Novel Diagnostic Technologies and Therapeutic Approaches _targeting Chronic Wound Biofilms and Microbiota.Curr Dermatol Rep. 2022 Jun;11(2):60-72. doi: 10.1007/s13671-022-00354-9. Epub 2022 Mar 25. Curr Dermatol Rep. 2022. PMID: 37007641 Free PMC article.
References
-
- Weidenmaier C, Kokai-Kun JF, Kulauzovic E, Kohler T, Thumm G, Stoll H, Götz F, Peschel A. Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int J Med Microbiol. 2008;298:505–513. doi: 10.1016/j.ijmm.2007.11.006. - DOI - PubMed
-
- Burian M, Rautenberg M, Kohler T, Fritz M, Krismer B, Unger C, Hoffmann WH, Peschel A, Wolz C, Goerke C. Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis. 2010;201:1414–1421. doi: 10.1086/651619. - DOI - PubMed
LinkOut - more resources
Full Text Sources
