Homotrimer cavin1 interacts with caveolin1 to facilitate tumor growth and activate microglia through extracellular vesicles in glioma
- PMID: 32550897
- PMCID: PMC7295042
- DOI: 10.7150/thno.45688
Homotrimer cavin1 interacts with caveolin1 to facilitate tumor growth and activate microglia through extracellular vesicles in glioma
Abstract
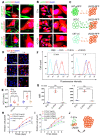
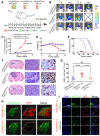
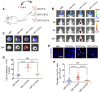
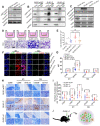
Background: Intercellular communication via extracellular vesicles (EVs) plays a critical role in glioma progression. However, little is known about the precise mechanism regulating EV secretion and function. Our previous study revealed that Cavin1 was positively correlated with malignancy grades of glioma patients, and that overexpressing Cavin1 in glioma cells enhanced the malignancy of nearby glioma cells via EVs. Methods: The current study used bioinformatics to design a variant Cavin1 (vCavin1) incapable of interacting with Caveolin1, and compared the effects of overexpressing Cavin1 and vCavin1 in glioma cells on EV production and function. Results: Remarkably, our results indicated that Cavin1 expression enhanced the secretion, uptake, and homing ability of glioma-derived EVs. EVs expressing Cavin1 promoted glioma growth in vitro and in vivo. In addition, Cavin1 expressing murine glioma cells recruited and activated microglia via EVs. However, vCavin1 neither was loaded onto EVs nor altered EV secretion and function. Conclusion: Our findings suggested that Cavin1-Caveolin1 interaction played a significant role in regulating production and function of glioma-EVs, and may act as a promising therapeutic _target in gliomas that express high levels of Cavin1.
Keywords: Cavin1-Caveolin1 interaction; extracellular vesicles; glioma; microglia.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Glioma-derived extracellular vesicles promote tumor progression by conveying WT1.Carcinogenesis. 2020 Sep 24;41(9):1238-1245. doi: 10.1093/carcin/bgaa052. Carcinogenesis. 2020. PMID: 32463428
-
Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain.Neuro Oncol. 2016 Jan;18(1):58-69. doi: 10.1093/neuonc/nov244. Epub 2015 Oct 3. Neuro Oncol. 2016. PMID: 26433199 Free PMC article.
-
Inflammation leads to distinct populations of extracellular vesicles from microglia.J Neuroinflammation. 2018 May 28;15(1):168. doi: 10.1186/s12974-018-1204-7. J Neuroinflammation. 2018. PMID: 29807527 Free PMC article.
-
Role of Extracellular Vesicles in Glioma Progression: Deciphering Cellular Biological Processes to Clinical Applications.Curr Top Med Chem. 2021;21(8):696-704. doi: 10.2174/1568026620666201207100139. Curr Top Med Chem. 2021. PMID: 33292136 Review.
-
Microglia-derived extracellular vesicles in Alzheimer's Disease: A double-edged sword.Biochem Pharmacol. 2018 Feb;148:184-192. doi: 10.1016/j.bcp.2017.12.020. Epub 2018 Jan 3. Biochem Pharmacol. 2018. PMID: 29305855 Review.
Cited by
-
EPIC-1042 as a potent PTRF/Cavin1-caveolin-1 interaction inhibitor to induce PARP1 autophagic degradation and suppress temozolomide efflux for glioblastoma.Neuro Oncol. 2024 Jan 5;26(1):100-114. doi: 10.1093/neuonc/noad159. Neuro Oncol. 2024. PMID: 37651725 Free PMC article.
-
Neuronal-driven glioma growth requires Gαi1 and Gαi3.Theranostics. 2021 Jul 25;11(17):8535-8549. doi: 10.7150/thno.61452. eCollection 2021. Theranostics. 2021. PMID: 34373757 Free PMC article.
-
Extracellular vesicles derived from hypoxic glioma stem-like cells confer temozolomide resistance on glioblastoma by delivering miR-30b-3p.Theranostics. 2021 Jan 1;11(4):1763-1779. doi: 10.7150/thno.47057. eCollection 2021. Theranostics. 2021. PMID: 33408780 Free PMC article.
-
Microglia and Brain Macrophages as Drivers of Glioma Progression.Int J Mol Sci. 2022 Dec 9;23(24):15612. doi: 10.3390/ijms232415612. Int J Mol Sci. 2022. PMID: 36555253 Free PMC article. Review.
-
Interactions between microglia and glioma in tumor microenvironment.Front Oncol. 2023 Aug 28;13:1236268. doi: 10.3389/fonc.2023.1236268. eCollection 2023. Front Oncol. 2023. PMID: 37700840 Free PMC article. Review.
References
-
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. - PubMed
-
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

