Applying Tissue Slice Culture in Cancer Research-Insights from Preclinical Proton Radiotherapy
- PMID: 32560230
- PMCID: PMC7352770
- DOI: 10.3390/cancers12061589
Applying Tissue Slice Culture in Cancer Research-Insights from Preclinical Proton Radiotherapy
Abstract
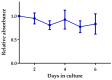
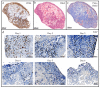
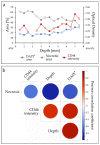
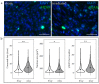
A challenge in cancer research is the definition of reproducible, reliable, and practical models, which reflect the effects of complex treatment modalities and the heterogeneous response of patients. Proton beam radiotherapy (PBRT), relative to conventional photon-based radiotherapy, offers the potential for iso-effective tumor control, while protecting the normal tissue surrounding the tumor. However, the effects of PBRT on the tumor microenvironment and the interplay with newly developed chemo- and immunotherapeutic approaches are still open for investigation. This work evaluated thin-cut tumor slice cultures (TSC) of head and neck cancer and organotypic brain slice cultures (OBSC) of adult mice brain, regarding their relevance for translational radiooncology research. TSC and OBSC were treated with PBRT and investigated for cell survival with a lactate dehydrogenase (LDH) assay, DNA repair via the DNA double strand break marker γH2AX, as well as histology with regards to morphology. Adult OBSC failed to be an appropriate model for radiobiological research questions. However, histological analysis of TSC showed DNA damage and tumor morphological results, comparable to known in vivo and in vitro data, making them a promising model to study novel treatment approaches in patient-derived xenografts or primary tumor material.
Keywords: DNA damage; head and neck cancer; organotypic brain slice culture; proton beam radiotherapy; thin-cut tissue slices; tumor biology.
Conflict of interest statement
In the past 5 years, M.K. received funding for her research projects by IBA (2016), Merck KGaA (2014–2018 for preclinical study; 2018–2020 for clinical study), Medipan GmbH (2014–2018). In the past 5 years, M.K. and S.L. have been involved in an ongoing publicly funded (German Federal Ministry of Education and Research) project with the companies Medipan, Attomol GmbH, GA Generic Assays GmbH, Gesellschaft für medizinische und wissenschaftliche genetische Analysen, Lipotype GmbH, and PolyAn GmbH (2019–2021). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The other authors declare no conflict of interest.
Figures


Similar articles
-
Patient derived ex vivo tissue slice cultures demonstrate a profound DNA double-strand break repair defect in HPV-positive oropharyngeal head and neck cancer.Radiother Oncol. 2022 Mar;168:138-146. doi: 10.1016/j.radonc.2022.01.017. Epub 2022 Jan 29. Radiother Oncol. 2022. PMID: 35093407
-
Culturing thick brain slices: an interstitial 3D microperfusion system for enhanced viability.J Neurosci Methods. 2009 Jun 15;180(2):243-54. doi: 10.1016/j.jneumeth.2009.03.016. Epub 2009 Mar 28. J Neurosci Methods. 2009. PMID: 19443039 Free PMC article.
-
Organotypic brain slice cultures of adult transgenic P301S mice--a model for tauopathy studies.PLoS One. 2012;7(9):e45017. doi: 10.1371/journal.pone.0045017. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984603 Free PMC article.
-
Models for Translational Proton Radiobiology-From Bench to Bedside and Back.Cancers (Basel). 2021 Aug 22;13(16):4216. doi: 10.3390/cancers13164216. Cancers (Basel). 2021. PMID: 34439370 Free PMC article. Review.
-
A Bridge Between in vitro and in vivo Studies in Neuroscience: Organotypic Brain Slice Cultures.Noro Psikiyatr Ars. 2020 Sep 21;57(4):333-337. doi: 10.29399/npa.26139. eCollection 2020 Dec. Noro Psikiyatr Ars. 2020. PMID: 33354128 Free PMC article. Review.
Cited by
-
Novel Ex Vivo Models of Epithelial Ovarian Cancer: The Future of Biomarker and Therapeutic Research.Front Oncol. 2022 Mar 25;12:837233. doi: 10.3389/fonc.2022.837233. eCollection 2022. Front Oncol. 2022. PMID: 35402223 Free PMC article. Review.
-
Patient-Derived Models of Cancer in the NCI PDMC Consortium: Selection, Pitfalls, and Practical Recommendations.Cancers (Basel). 2024 Jan 29;16(3):565. doi: 10.3390/cancers16030565. Cancers (Basel). 2024. PMID: 38339316 Free PMC article. Review.
-
Lung Organotypic Slices Enable Rapid Quantification of Acute Radiotherapy Induced Toxicity.Cells. 2023 Oct 11;12(20):2435. doi: 10.3390/cells12202435. Cells. 2023. PMID: 37887279 Free PMC article.
-
Combined Systemic Drug Treatment with Proton Therapy: Investigations on Patient-Derived Organoids.Cancers (Basel). 2022 Aug 3;14(15):3781. doi: 10.3390/cancers14153781. Cancers (Basel). 2022. PMID: 35954444 Free PMC article.
-
Late Side Effects in Normal Mouse Brain Tissue After Proton Irradiation.Front Oncol. 2021 Jan 11;10:598360. doi: 10.3389/fonc.2020.598360. eCollection 2020. Front Oncol. 2021. PMID: 33520710 Free PMC article.
References
-
- Barnes B., Kraywinkel K., Nowossadeck E., Schönfeld I., Starker A., Wienecke A., Wolf U. Bericht zum Krebsgeschehen in Deutschland 2016. Robert Koch-Institut; Berlin, Germany: 2016. p. 274. - DOI
-
- Moreno A.C., Frank S.J., Garden A.S., Rosenthal D.I., Fuller C.D., Gunn G.B., Reddy J.P., Morrison W.H., Williamson T.D., Holliday E.B., et al. Intensity modulated proton therapy (IMPT)—The future of IMRT for head and neck cancer. Oral Oncol. 2019;88:66–74. doi: 10.1016/j.oraloncology.2018.11.015. - DOI - PMC - PubMed
-
- PTCOG—Home. [(accessed on 5 March 2020)]; Available online: https://www.ptcog.ch/index.php.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

