Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease
- PMID: 32615709
- PMCID: PMC7386127
- DOI: 10.3803/EnM.2020.35.2.243
Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease
Abstract
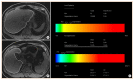
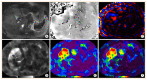
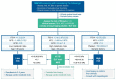
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent liver diseases and can progress to advanced fibrosis and end-stage liver disease. Thus, intensive research has been performed to develop noninvasive methods for the diagnosis of nonalcoholic steatohepatitis (NASH) and fibrosis. Currently, no single noninvasive tool covers all of the stages of pathologies and conditions of NAFLD, and the cost and feasibility of known techniques are also important issues. Blood biomarkers for NAFLD may be useful to select subjects who need ultrasonography (US) screening for NAFLD, and noninvasive tools for assessing fibrosis may be helpful to exclude the probability of significant fibrosis and to predict advanced fibrosis, thus guiding the decision of whether to perform liver biopsy in patients with NAFLD. Among various methods, magnetic resonance-based methods have been shown to perform better than other methods in assessing steatosis as well as in detecting hepatic fibrosis. Many genetic markers are associated with the development and progression of NAFLD. Further well-designed studies are needed to determine which biomarker panels, imaging studies, genetic marker panels, or combinations thereof perform well for diagnosing NAFLD, differentiating NASH and fibrosis, and following-up NAFLD, respectively.
Keywords: Biomarkers; Fibrosis; Liver steatosis; Non-alcoholic fatty liver disease; Evaluation.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease.Gastroenterology. 2019 Apr;156(5):1264-1281.e4. doi: 10.1053/j.gastro.2018.12.036. Epub 2019 Jan 18. Gastroenterology. 2019. PMID: 30660725 Free PMC article. Review.
-
Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice.World J Gastroenterol. 2019 Mar 21;25(11):1307-1326. doi: 10.3748/wjg.v25.i11.1307. World J Gastroenterol. 2019. PMID: 30918425 Free PMC article. Review.
-
Current and Emerging Biomarkers and Imaging Modalities for Nonalcoholic Fatty Liver Disease: Clinical and Research Applications.Clin Ther. 2021 Sep;43(9):1505-1522. doi: 10.1016/j.clinthera.2021.07.012. Epub 2021 Aug 13. Clin Ther. 2021. PMID: 34400007 Review.
-
Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.World J Gastroenterol. 2014 Jan 14;20(2):475-85. doi: 10.3748/wjg.v20.i2.475. World J Gastroenterol. 2014. PMID: 24574716 Free PMC article. Review.
-
Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Noninvasive Biomarkers and Transient Elastography.Clin Liver Dis. 2018 Feb;22(1):73-92. doi: 10.1016/j.cld.2017.08.004. Clin Liver Dis. 2018. PMID: 29128062 Review.
Cited by
-
The Leg Fat to Total Fat Ratio Is Associated with Lower Risks of Non-Alcoholic Fatty Liver Disease and Less Severe Hepatic Fibrosis: Results from Nationwide Surveys (KNHANES 2008-2011).Endocrinol Metab (Seoul). 2021 Dec;36(6):1232-1242. doi: 10.3803/EnM.2021.1087. Epub 2021 Nov 23. Endocrinol Metab (Seoul). 2021. PMID: 34809412 Free PMC article.
-
Triglyceride Glucose-Waist Circumference Is Superior to the Homeostasis Model Assessment of Insulin Resistance in Identifying Nonalcoholic Fatty Liver Disease in Healthy Subjects.J Clin Med. 2021 Dec 23;11(1):41. doi: 10.3390/jcm11010041. J Clin Med. 2021. PMID: 35011784 Free PMC article.
-
Plasma Metabolomics and Machine Learning-Driven Novel Diagnostic Signature for Non-Alcoholic Steatohepatitis.Biomedicines. 2022 Jul 11;10(7):1669. doi: 10.3390/biomedicines10071669. Biomedicines. 2022. PMID: 35884973 Free PMC article.
-
Non-Laboratory-Based Simple Screening Model for Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Developed Using Multi-Center Cohorts.Endocrinol Metab (Seoul). 2021 Aug;36(4):823-834. doi: 10.3803/EnM.2021.1074. Epub 2021 Aug 27. Endocrinol Metab (Seoul). 2021. PMID: 34474517 Free PMC article.
-
Accuracy of FIB-4 to Detect Elevated Liver Stiffness Measurements in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study in Referral Centers.Int J Mol Sci. 2022 Oct 18;23(20):12489. doi: 10.3390/ijms232012489. Int J Mol Sci. 2022. PMID: 36293345 Free PMC article.
References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21(1 Pt 1):138–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

