In Vitro Mechanical and Biological Properties of 3D Printed Polymer Composite and β-Tricalcium Phosphate Scaffold on Human Dental Pulp Stem Cells
- PMID: 32650530
- PMCID: PMC7412522
- DOI: 10.3390/ma13143057
In Vitro Mechanical and Biological Properties of 3D Printed Polymer Composite and β-Tricalcium Phosphate Scaffold on Human Dental Pulp Stem Cells
Abstract
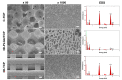
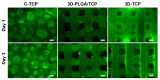
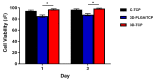
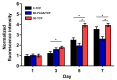
3D printed biomaterials have been extensively investigated and developed in the field of bone regeneration related to clinical issues. However, specific applications of 3D printed biomaterials in different dental areas have seldom been reported. In this study, we aimed to and successfully fabricated 3D poly (lactic-co-glycolic acid)/β-tricalcium phosphate (3D-PLGA/TCP) and 3D β-tricalcium phosphate (3D-TCP) scaffolds using two relatively distinct 3D printing (3DP) technologies. Conjunctively, we compared and investigated mechanical and biological responses on human dental pulp stem cells (hDPSCs). Physicochemical properties of the scaffolds, including pore structure, chemical elements, and compression modulus, were characterized. hDPSCs were cultured on scaffolds for subsequent investigations of biocompatibility and osteoconductivity. Our findings indicate that 3D printed PLGA/TCP and β-tricalcium phosphate (β-TCP) scaffolds possessed a highly interconnected and porous structure. 3D-TCP scaffolds exhibited better compressive strength than 3D-PLGA/TCP scaffolds, while the 3D-PLGA/TCP scaffolds revealed a flexible mechanical performance. The introduction of 3D structure and β-TCP components increased the adhesion and proliferation of hDPSCs and promoted osteogenic differentiation. In conclusion, 3D-PLGA/TCP and 3D-TCP scaffolds, with the incorporation of hDPSCs as a personalized restoration approach, has a prospective potential to repair minor and critical bone defects in oral and maxillofacial surgery, respectively.
Keywords: 3D printing; bone regeneration; ceramic printing; dental biomaterials; human dental pulp stem cell; in vitro research; polymer printing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
3D printed polycaprolactone/β-tricalcium phosphate/carbon nanotube composite - Physical properties and biocompatibility.Heliyon. 2024 Feb 20;10(5):e26071. doi: 10.1016/j.heliyon.2024.e26071. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38468962 Free PMC article.
-
Collagenous matrix supported by a 3D-printed scaffold for osteogenic differentiation of dental pulp cells.Dent Mater. 2018 Feb;34(2):209-220. doi: 10.1016/j.dental.2017.10.001. Epub 2017 Oct 18. Dent Mater. 2018. PMID: 29054688 Free PMC article.
-
[Biological evaluation of three-dimensional printed co-poly lactic acid/glycolic acid/tri-calcium phosphate scaffold for bone reconstruction].Zhonghua Kou Qiang Yi Xue Za Zhi. 2016 Nov 9;51(11):661-666. doi: 10.3760/cma.j.issn.1002-0098.2016.11.005. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016. PMID: 27806758 Chinese.
-
3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review.Materials (Basel). 2022 Apr 2;15(7):2621. doi: 10.3390/ma15072621. Materials (Basel). 2022. PMID: 35407950 Free PMC article. Review.
-
Tissue regeneration therapy by Nano composite scaffolds based on PLGA hydrogel embedded with human dental pulp stem cells: a systematic review.Am J Transl Res. 2024 May 15;16(5):1521-1530. doi: 10.62347/QBKO6417. eCollection 2024. Am J Transl Res. 2024. PMID: 38883345 Free PMC article. Review.
Cited by
-
Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering.Polymers (Basel). 2022 Feb 11;14(4):669. doi: 10.3390/polym14040669. Polymers (Basel). 2022. PMID: 35215595 Free PMC article.
-
Additive Manufacturing Processes in Medical Applications.Materials (Basel). 2021 Jan 3;14(1):191. doi: 10.3390/ma14010191. Materials (Basel). 2021. PMID: 33401601 Free PMC article. Review.
-
Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration.Polymers (Basel). 2020 Dec 1;12(12):2881. doi: 10.3390/polym12122881. Polymers (Basel). 2020. PMID: 33271770 Free PMC article. Review.
-
Bioactive ceramic-based materials: beneficial properties and potential applications in dental repair and regeneration.Regen Med. 2024 May 3;19(5):257-278. doi: 10.1080/17460751.2024.2343555. Epub 2024 May 22. Regen Med. 2024. PMID: 39118532 Review.
-
The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells.Materials (Basel). 2022 Mar 15;15(6):2170. doi: 10.3390/ma15062170. Materials (Basel). 2022. PMID: 35329621 Free PMC article.
References
-
- Cao S., Zhang L., Chen Z., Li S., Jiang H., Tian Y., Gu W., Zhou M., Chen X. The Effect and Biocompatibility of Nanofibrous Nano-Hydroxyapatite/Polycaprolactone/Gelatin Scaffolds Multipurpose Membrane in Guiding Bone Regeneration. J. Biomater. Tissue Eng. 2017;7:721–729. doi: 10.1166/jbt.2017.1619. - DOI
-
- Zhou M., Peng X., Mao C., Xu F., Hu M., Yu G.Y. Primate mandibular reconstruction with prefabricated, vascularized tissue-engineered bone flaps and recombinant human bone morphogenetic protein-2 implanted in situ. Biomaterials. 2010;31:4935–4943. doi: 10.1016/j.biomaterials.2010.02.072. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

