Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling
- PMID: 32669546
- PMCID: PMC7363928
- DOI: 10.1038/s41467-020-17402-2
Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling
Abstract
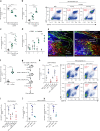
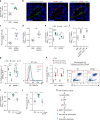
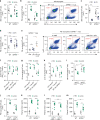
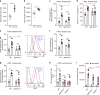
Neutrophils provide first line of host defense against bacterial infections utilizing glycolysis for their effector functions. How glycolysis and its major byproduct lactate are triggered in bone marrow (BM) neutrophils and their contribution to neutrophil mobilization in acute inflammation is not clear. Here we report that bacterial lipopolysaccharides (LPS) or Salmonella Typhimurium triggers lactate release by increasing glycolysis, NADPH-oxidase-mediated reactive oxygen species and HIF-1α levels in BM neutrophils. Increased release of BM lactate preferentially promotes neutrophil mobilization by reducing endothelial VE-Cadherin expression, increasing BM vascular permeability via endothelial lactate-receptor GPR81 signaling. GPR81-/- mice mobilize reduced levels of neutrophils in response to LPS, unless rescued by VE-Cadherin disrupting antibodies. Lactate administration also induces release of the BM neutrophil mobilizers G-CSF, CXCL1 and CXCL2, indicating that this metabolite drives neutrophil mobilization via multiple pathways. Our study reveals a metabolic crosstalk between lactate-producing neutrophils and BM endothelium, which controls neutrophil mobilization under bacterial infection.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands.Blood. 2011 Apr 21;117(16):4349-57. doi: 10.1182/blood-2010-09-308387. Epub 2011 Jan 11. Blood. 2011. PMID: 21224471 Free PMC article.
-
CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow.J Clin Invest. 2010 Jul;120(7):2423-31. doi: 10.1172/JCI41649. J Clin Invest. 2010. PMID: 20516641 Free PMC article.
-
Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner.PLoS One. 2016 Nov 15;11(11):e0163694. doi: 10.1371/journal.pone.0163694. eCollection 2016. PLoS One. 2016. PMID: 27846210 Free PMC article.
-
Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance.Front Cell Infect Microbiol. 2017 Aug 25;7:373. doi: 10.3389/fcimb.2017.00373. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28890882 Free PMC article. Review.
-
Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon.Pharmacol Ther. 2020 Feb;206:107451. doi: 10.1016/j.pharmthera.2019.107451. Epub 2019 Dec 10. Pharmacol Ther. 2020. PMID: 31836453 Review.
Cited by
-
Lactylation, an emerging hallmark of metabolic reprogramming: Current progress and open challenges.Front Cell Dev Biol. 2022 Aug 26;10:972020. doi: 10.3389/fcell.2022.972020. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092712 Free PMC article. Review.
-
Activation induces shift in nutrient utilization that differentially impacts cell functions in human neutrophils.Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2321212121. doi: 10.1073/pnas.2321212121. Epub 2024 Sep 16. Proc Natl Acad Sci U S A. 2024. PMID: 39284072
-
Differential Metabotypes in Synovial Fibroblasts and Synovial Fluid in Hip Osteoarthritis Patients Support Inflammatory Responses.Int J Mol Sci. 2022 Mar 17;23(6):3266. doi: 10.3390/ijms23063266. Int J Mol Sci. 2022. PMID: 35328687 Free PMC article.
-
The Metabolic Syndrome, a Human Disease.Int J Mol Sci. 2024 Feb 13;25(4):2251. doi: 10.3390/ijms25042251. Int J Mol Sci. 2024. PMID: 38396928 Free PMC article. Review.
-
Metformin Attenuates Neutrophil Recruitment through the H3K18 Lactylation/Reactive Oxygen Species Pathway in Zebrafish.Antioxidants (Basel). 2024 Jan 30;13(2):176. doi: 10.3390/antiox13020176. Antioxidants (Basel). 2024. PMID: 38397774 Free PMC article.
References
-
- Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. - PubMed
-
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 2012;30:459–489. - PubMed
-
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

