Modeling neoplastic disease with spheroids and organoids
- PMID: 32677979
- PMCID: PMC7364537
- DOI: 10.1186/s13045-020-00931-0
Modeling neoplastic disease with spheroids and organoids
Abstract
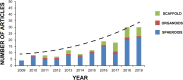
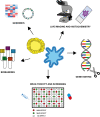
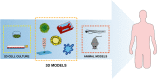
Cancer is a complex disease in which both genetic defects and microenvironmental components contribute to the development, progression, and metastasization of disease, representing major hurdles in the identification of more effective and safer treatment regimens for patients. Three-dimensional (3D) models are changing the paradigm of preclinical cancer research as they more closely resemble the complex tissue environment and architecture found in clinical tumors than in bidimensional (2D) cell cultures. Among 3D models, spheroids and organoids represent the most versatile and promising models in that they are capable of recapitulating the heterogeneity and pathophysiology of human cancers and of filling the gap between conventional 2D in vitro testing and animal models. Such 3D systems represent a powerful tool for studying cancer biology, enabling us to model the dynamic evolution of neoplastic disease from the early stages to metastatic dissemination and the interactions with the microenvironment. Spheroids and organoids have recently been used in the field of drug discovery and personalized medicine. The combined use of 3D models could potentially improve the robustness and reliability of preclinical research data, reducing the need for animal testing and favoring their transition to clinical practice. In this review, we summarize the recent advances in the use of these 3D systems for cancer modeling, focusing on their innovative translational applications, looking at future challenges, and comparing them with most widely used animal models.
Keywords: 3D models; Cancer; Drug discovery; Organoid; Spheroid; Tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
[Spheroids to organoids: Solid cancer models for anticancer drug discovery].Bull Cancer. 2022 Jan;109(1):49-57. doi: 10.1016/j.bulcan.2021.09.019. Epub 2021 Nov 27. Bull Cancer. 2022. PMID: 34848046 Review. French.
-
Application of Cancer Organoid Model for Drug Screening and Personalized Therapy.Cells. 2019 May 17;8(5):470. doi: 10.3390/cells8050470. Cells. 2019. PMID: 31108870 Free PMC article. Review.
-
Sarcoma Spheroids and Organoids-Promising Tools in the Era of Personalized Medicine.Int J Mol Sci. 2018 Feb 21;19(2):615. doi: 10.3390/ijms19020615. Int J Mol Sci. 2018. PMID: 29466296 Free PMC article. Review.
-
[Progress in prostate cancer study: 3D cell culture enables the ex vivo reproduction of tumor characteristics].Presse Med. 2017 Oct;46(10):954-965. doi: 10.1016/j.lpm.2017.06.014. Epub 2017 Sep 28. Presse Med. 2017. PMID: 28967525 French.
-
3D Biomimetic Models to Reconstitute Tumor Microenvironment In Vitro: Spheroids, Organoids, and Tumor-on-a-Chip.Adv Healthc Mater. 2023 Jul;12(18):e2202609. doi: 10.1002/adhm.202202609. Epub 2023 Mar 29. Adv Healthc Mater. 2023. PMID: 36917657 Free PMC article. Review.
Cited by
-
Patient-Derived Organoids as a Model for Cancer Drug Discovery.Int J Mol Sci. 2021 Mar 27;22(7):3483. doi: 10.3390/ijms22073483. Int J Mol Sci. 2021. PMID: 33801782 Free PMC article. Review.
-
Three dimensional models of dedifferentiated liposarcoma cell lines: scaffold-based and scaffold-free approaches.Hum Cell. 2023 May;36(3):1081-1089. doi: 10.1007/s13577-023-00865-y. Epub 2023 Feb 10. Hum Cell. 2023. PMID: 36763259
-
3D Models of Cellular Spheroids As a Universal Tool for Studying the Cytotoxic Properties of Anticancer Compounds In Vitro.Acta Naturae. 2022 Jan-Mar;14(1):92-100. doi: 10.32607/actanaturae.11603. Acta Naturae. 2022. PMID: 35441052 Free PMC article.
-
A Simple and Fast Method for the Formation and Downstream Processing of Cancer-Cell-Derived 3D Spheroids: An Example Using Nicotine-Treated A549 Lung Cancer 3D Spheres.Methods Protoc. 2023 Oct 4;6(5):94. doi: 10.3390/mps6050094. Methods Protoc. 2023. PMID: 37888026 Free PMC article.
-
Regulation of cell fate by cell imprinting approach in vitro.Bioimpacts. 2024;14(3):29945. doi: 10.34172/bi.2023.29945. Epub 2023 Nov 28. Bioimpacts. 2024. PMID: 38938752 Free PMC article. Review.
References
-
- Zanoni M, Pignatta S, Arienti C, Bonafè M, Tesei A. Anticancer drug discovery using multicellular tumor spheroid models. Expert Opin Drug Discov. 2019;14:289–301. - PubMed
-
- Hutchinson L, Kirk R. High drug attrition rates—Where are we going wrong. Nat Rev Clin Oncol. 2011;8:189–190. - PubMed
-
- Caponigro G, Sellers WR. Advances in the preclinical testing of cancer therapeutic hypotheses. Nat Rev Drug Discov. 2011;10:179–187. - PubMed
-
- Nass SJ, Rothenberg ML, Pentz R, Hricak H, Abernethy A, Anderson K, et al. Accelerating anticancer drug development - opportunities and trade-offs. Nat Rev Clin Oncol. 2018;15:777–786. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

