Hepatocyte expression of the micropeptide adropin regulates the liver fasting response and is enhanced by caloric restriction
- PMID: 32727846
- PMCID: PMC7535914
- DOI: 10.1074/jbc.RA120.014381
Hepatocyte expression of the micropeptide adropin regulates the liver fasting response and is enhanced by caloric restriction
Abstract
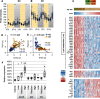
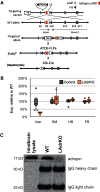
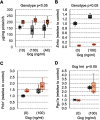
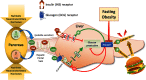
The micropeptide adropin encoded by the clock-controlled energy homeostasis-associated gene is implicated in the regulation of glucose metabolism. However, its links to rhythms of nutrient intake, energy balance, and metabolic control remain poorly defined. Using surveys of Gene Expression Omnibus data sets, we confirm that fasting suppresses liver adropin expression in lean C57BL/6J (B6) mice. However, circadian rhythm data are inconsistent. In lean mice, caloric restriction (CR) induces bouts of compulsive binge feeding separated by prolonged fasting intervals, increasing NAD-dependent deacetylase sirtuin-1 signaling important for glucose and lipid metabolism regulation. CR up-regulates adropin expression and induces rhythms correlating with cellular stress-response pathways. Furthermore, adropin expression correlates positively with phosphoenolpyruvate carboxokinase-1 (Pck1) expression, suggesting a link with gluconeogenesis. Our previous data suggest that adropin suppresses gluconeogenesis in hepatocytes. Liver-specific adropin knockout (LAdrKO) mice exhibit increased glucose excursions following pyruvate injections, indicating increased gluconeogenesis. Gluconeogenesis is also increased in primary cultured hepatocytes derived from LAdrKO mice. Analysis of circulating insulin levels and liver expression of fasting-responsive cAMP-dependent protein kinase A (PKA) signaling pathways also suggests enhanced responses in LAdrKO mice during a glucagon tolerance test (250 µg/kg intraperitoneally). Fasting-associated changes in PKA signaling are attenuated in transgenic mice constitutively expressing adropin and in fasting mice treated acutely with adropin peptide. In summary, hepatic adropin expression is regulated by nutrient- and clock-dependent extrahepatic signals. CR induces pronounced postprandial peaks in hepatic adropin expression. Rhythms of hepatic adropin expression appear to link energy balance and cellular stress to the intracellular signal transduction pathways that drive the liver fasting response.
Keywords: circadian rhythm; diet; glucagon; glucose metabolism; hepatocyte; insulin; peptide hormone; signal transduction; stress response.
© 2020 Banerjee et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures





Similar articles
-
Adropin's Role in Energy Homeostasis and Metabolic Disorders.Int J Mol Sci. 2022 Jul 28;23(15):8318. doi: 10.3390/ijms23158318. Int J Mol Sci. 2022. PMID: 35955453 Free PMC article. Review.
-
The peptide hormone adropin regulates signal transduction pathways controlling hepatic glucose metabolism in a mouse model of diet-induced obesity.J Biol Chem. 2019 Sep 6;294(36):13366-13377. doi: 10.1074/jbc.RA119.008967. Epub 2019 Jul 19. J Biol Chem. 2019. PMID: 31324719 Free PMC article.
-
Regulation of hepatic gluconeogenesis by nuclear factor Y transcription factor in mice.J Biol Chem. 2018 May 18;293(20):7894-7904. doi: 10.1074/jbc.RA117.000508. Epub 2018 Mar 12. J Biol Chem. 2018. PMID: 29530977 Free PMC article.
-
Zbtb7c is a critical gluconeogenic transcription factor that induces glucose-6-phosphatase and phosphoenylpyruvate carboxykinase 1 genes expression during mice fasting.Biochim Biophys Acta Gene Regul Mech. 2019 Jun;1862(6):643-656. doi: 10.1016/j.bbagrm.2019.04.001. Epub 2019 Apr 5. Biochim Biophys Acta Gene Regul Mech. 2019. PMID: 30959128
-
Calorie restriction enhances the expression of key metabolic enzymes associated with protein renewal during aging.Ann N Y Acad Sci. 2001 Apr;928:296-304. doi: 10.1111/j.1749-6632.2001.tb05659.x. Ann N Y Acad Sci. 2001. PMID: 11795521 Review.
Cited by
-
Adropin's Role in Energy Homeostasis and Metabolic Disorders.Int J Mol Sci. 2022 Jul 28;23(15):8318. doi: 10.3390/ijms23158318. Int J Mol Sci. 2022. PMID: 35955453 Free PMC article. Review.
-
Diet-induced obese mice are resistant to improvements in cardiac function resulting from short-term adropin treatment.Curr Res Physiol. 2022 Jan 25;5:55-62. doi: 10.1016/j.crphys.2022.01.005. eCollection 2022. Curr Res Physiol. 2022. PMID: 35128468 Free PMC article.
-
New Peptides as Potential Players in the Crosstalk Between the Brain and Obesity, Metabolic and Cardiovascular Diseases.Front Physiol. 2021 Aug 23;12:692642. doi: 10.3389/fphys.2021.692642. eCollection 2021. Front Physiol. 2021. PMID: 34497533 Free PMC article. Review.
-
Low circulating adropin levels in late-middle aged African Americans with poor cognitive performance.NPJ Aging. 2023 Nov 9;9(1):24. doi: 10.1038/s41514-023-00122-4. NPJ Aging. 2023. PMID: 37945652 Free PMC article.
-
Hepatokines: unveiling the molecular and cellular mechanisms connecting hepatic tissue to insulin resistance and inflammation.Acta Diabetol. 2024 Nov;61(11):1339-1361. doi: 10.1007/s00592-024-02335-9. Epub 2024 Jul 20. Acta Diabetol. 2024. PMID: 39031190 Review.
References
-
- Carvunis A. R., Rolland T., Wapinski I., Calderwood M. A., Yildirim M. A., Simonis N., Charloteaux B., Hidalgo C. A., Barbette J., Santhanam B., Brar G. A., Weissman J. S., Regev A., Thierry-Mieg N., Cusick M. E., et al. (2012) Proto-genes and de novo gene birth. Nature 487, 370–374 10.1038/nature11184 - DOI - PMC - PubMed
-
- Kumar K. G., Trevaskis J. L., Lam D. D., Sutton G. M., Koza R. A., Chouljenko V. N., Kousoulas K. G., Rogers P. M., Kesterson R. A., Thearle M., Ferrante A. W. Jr., Mynatt R. L., Burris T. P., Dong J. Z., Halem H. A., et al. (2008) Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 8, 468–481 10.1016/j.cmet.2008.10.011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

