TLR7 Stimulation With Imiquimod Induces Selective Autophagy and Controls Mycobacterium tuberculosis Growth in Mouse Macrophages
- PMID: 32765474
- PMCID: PMC7380068
- DOI: 10.3389/fmicb.2020.01684
TLR7 Stimulation With Imiquimod Induces Selective Autophagy and Controls Mycobacterium tuberculosis Growth in Mouse Macrophages
Abstract
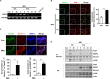
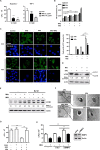
Autophagy is a lysosomal self-digestion pathway that maintains internal homeostasis inside cells and critical process by which the innate immune system eliminates intracellular bacteria. In this study, we showed that stimulation of toll-like receptor 7 (TLR7) with imiquimod (IMQ) triggered autophagic cell death in macrophages by enhancing the generation of reactive oxygen species (ROS) via the p38- or MEK/ERK1/2-mediated signaling pathway in the early phase. IMQ significantly increased mitochondrial ROS and _targeted autophagosomes to the mitochondria. Stimulation of TLR7 with IMQ enhanced the expression of BNIP3, which was localized to mitochondria and interacted with beclin-1, leading to mitophagy. In addition, IMQ substantially induced NO production through the GSK-3β-mediated signaling pathway, which led to autophagy in the late stage. We further examined whether the induction of autophagy by IMQ effectively eliminated intracellular microbes. Macrophages were infected with a virulent Mycobacterium tuberculosis (Mtb) strain, H37Rv, and then treated with IMQ. IMQ suppressed intracellular Mtb growth by inducing autophagy in a dose-dependent manner and increased NO production. Inhibition of autophagy using 3-methyladenine (3-MA) prevented autophagosome formation and control of intracellular Mtb growth in macrophages. These findings revealed a novel mechanism by which IMQ induces selective autophagy to promote intracellular killing machinery against Mtb infection in macrophages.
Keywords: Mycobacterium tuberculosis; autophagosome; imiquimod (IMQ); mitophagy; mycobactericidal activity; toll-like receptor 7 (TLR7).
Copyright © 2020 Lee, Kang, Woo, Hahn, Ko and Jung.
Figures

Similar articles
-
Imiquimod-induced autophagy is regulated by ER stress-mediated PKR activation in cancer cells.J Dermatol Sci. 2017 Aug;87(2):138-148. doi: 10.1016/j.jdermsci.2017.04.011. Epub 2017 May 2. J Dermatol Sci. 2017. PMID: 28601430
-
The TLR7 agonist imiquimod induces anti-cancer effects via autophagic cell death and enhances anti-tumoral and systemic immunity during radiotherapy for melanoma.Onco_target. 2017 Apr 11;8(15):24932-24948. doi: 10.18632/onco_target.15326. Onco_target. 2017. PMID: 28212561 Free PMC article.
-
Imiquimod-induced ROS production disrupts the balance of mitochondrial dynamics and increases mitophagy in skin cancer cells.J Dermatol Sci. 2020 Jun;98(3):152-162. doi: 10.1016/j.jdermsci.2020.03.009. Epub 2020 Apr 23. J Dermatol Sci. 2020. PMID: 32376151
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
[Protective immunity against Mycobacterium tuberculosis].Kekkaku. 2006 Nov;81(11):687-91. Kekkaku. 2006. PMID: 17154048 Review. Japanese.
Cited by
-
Recognition of Mycobacterium tuberculosis by macrophage Toll-like receptor and its role in autophagy.Inflamm Res. 2024 May;73(5):753-770. doi: 10.1007/s00011-024-01864-x. Epub 2024 Apr 2. Inflamm Res. 2024. PMID: 38563966 Review.
-
Activation of TLR7-mediated autophagy increases epileptic susceptibility via reduced KIF5A-dependent GABAA receptor transport in a murine model.Exp Mol Med. 2023 Jun;55(6):1159-1173. doi: 10.1038/s12276-023-01000-5. Epub 2023 Jun 1. Exp Mol Med. 2023. PMID: 37258573 Free PMC article.
-
Host-Directed Therapies for Tuberculosis.Pathogens. 2022 Nov 3;11(11):1291. doi: 10.3390/pathogens11111291. Pathogens. 2022. PMID: 36365041 Free PMC article. Review.
-
Identification of Potential Diagnoses Based on Immune Infiltration and Autophagy Characteristics in Major Depressive Disorder.Front Genet. 2022 Apr 26;13:702366. doi: 10.3389/fgene.2022.702366. eCollection 2022. Front Genet. 2022. PMID: 35559009 Free PMC article.
-
To eat or not to eat mitochondria? How do host cells cope with mitophagy upon bacterial infection?PLoS Pathog. 2023 Jul 6;19(7):e1011471. doi: 10.1371/journal.ppat.1011471. eCollection 2023 Jul. PLoS Pathog. 2023. PMID: 37410705 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

