Dual _targeting of hepatic fibrosis and atherogenesis by icosabutate, an engineered eicosapentaenoic acid derivative
- PMID: 32841505
- PMCID: PMC7702170
- DOI: 10.1111/liv.14643
Dual _targeting of hepatic fibrosis and atherogenesis by icosabutate, an engineered eicosapentaenoic acid derivative
Abstract
Background & aims: While fibrosis stage predicts liver-associated mortality, cardiovascular disease (CVD) is still the major overall cause of mortality in patients with NASH. Novel NASH drugs should thus ideally reduce both liver fibrosis and CVD. Icosabutate is a semi-synthetic, liver-_targeted eicosapentaenoic acid (EPA) derivative in clinical development for NASH. The primary aims of the current studies were to establish both the anti-fibrotic and anti-atherogenic efficacy of icosabutate in conjunction with changes in lipotoxic and atherogenic lipids in liver and plasma respectively.
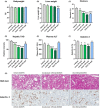
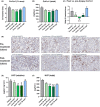
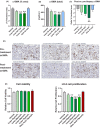
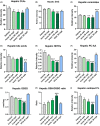
Methods: The effects of icosabutate on fibrosis progression and lipotoxicity were investigated in amylin liver NASH (AMLN) diet (high fat, cholesterol and fructose) fed ob/ob mice with biopsy-confirmed steatohepatitis and fibrosis and compared with the activity of obeticholic acid. APOE*3Leiden.CETP mice, a translational model for hyperlipidaemia and atherosclerosis, were used to evaluate the mechanisms underlying the lipid-lowering effect of icosabutate and its effect on atherosclerosis.
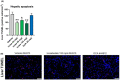
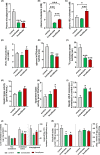
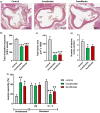
Results: In AMLN ob/ob mice, icosabutate significantly reduced hepatic fibrosis and myofibroblast content in association with downregulation of the arachidonic acid cascade and a reduction in both hepatic oxidised phospholipids and apoptosis. In APOE*3Leiden.CETP mice, icosabutate reduced plasma cholesterol and TAG levels via increased hepatic uptake, upregulated hepatic lipid metabolism and downregulated inflammation pathways, and effectively decreased atherosclerosis development.
Conclusions: Icosabutate, a structurally engineered EPA derivative, effectively attenuates both hepatic fibrosis and atherogenesis and offers an attractive therapeutic approach to both liver- and CV-related morbidity and mortality in NASH patients.
Keywords: NASH; apoptosis; arachidonic acid; atherosclerosis; lipotoxicity; oxidised phospholipids.
© 2020 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
Northsea Therapeutics BV acquired the commercial rights for icosabutate. JK and SF are paid consultants and have stock options in Northsea Therapeutics BV. DF and TS are employees of NorthSea Therapeutics BV but had no role in data collection. SF acts as a paid consultant for the following companies: 89 Bio, Amgen, Axcella Health, Blade Therapeutics, Bristol Myers Squibb, Can‐Fite Biopharma, ChemomAb, Escient Pharmaceuticals, Forbion, Galmed, Gordian Biotechnology, Glycotest, Glympse Bio, In sitro, Morphic Therapeutics, Novartis, Ono Pharmaceuticals, Scholar Rock, Surrozen. All other authors have no conflict of interest to declare.
Figures

Similar articles
-
A structurally engineered fatty acid, icosabutate, suppresses liver inflammation and fibrosis in NASH.J Hepatol. 2022 Apr;76(4):800-811. doi: 10.1016/j.jhep.2021.12.004. Epub 2021 Dec 13. J Hepatol. 2022. PMID: 34915054
-
Icosabutate Exerts Beneficial Effects Upon Insulin Sensitivity, Hepatic Inflammation, Lipotoxicity, and Fibrosis in Mice.Hepatol Commun. 2019 Dec 24;4(2):193-207. doi: 10.1002/hep4.1453. eCollection 2020 Feb. Hepatol Commun. 2019. PMID: 32025605 Free PMC article.
-
Towards a standard diet-induced and biopsy-confirmed mouse model of non-alcoholic steatohepatitis: Impact of dietary fat source.World J Gastroenterol. 2019 Sep 7;25(33):4904-4920. doi: 10.3748/wjg.v25.i33.4904. World J Gastroenterol. 2019. PMID: 31543682 Free PMC article.
-
Nonalcoholic Steatohepatitis (NASH) and Hepatic Fibrosis: Emerging Therapies.Annu Rev Pharmacol Toxicol. 2018 Jan 6;58:649-662. doi: 10.1146/annurev-pharmtox-010617-052545. Epub 2017 Oct 20. Annu Rev Pharmacol Toxicol. 2018. PMID: 29058997 Review.
-
New Drugs for Hepatic Fibrosis.Front Pharmacol. 2022 Jun 13;13:874408. doi: 10.3389/fphar.2022.874408. eCollection 2022. Front Pharmacol. 2022. PMID: 35770089 Free PMC article. Review.
Cited by
-
Beneficial effects of elafibranor on NASH in E3L.CETP mice and differences between mice and men.Sci Rep. 2021 Mar 3;11(1):5050. doi: 10.1038/s41598-021-83974-8. Sci Rep. 2021. PMID: 33658534 Free PMC article.
-
Protective Mechanism of Nostoc sphaeroides Kütz. Polysaccharide on Liver Fibrosis by HFD-Induced Liver Fat Synthesis and Oxidative Stress.Evid Based Complement Alternat Med. 2022 Jul 5;2022:1745244. doi: 10.1155/2022/1745244. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35836833 Free PMC article.
-
Therapeutic and diagnostic _targeting of fibrosis in metabolic, proliferative and viral disorders.Adv Drug Deliv Rev. 2021 Aug;175:113831. doi: 10.1016/j.addr.2021.113831. Epub 2021 Jun 15. Adv Drug Deliv Rev. 2021. PMID: 34139255 Free PMC article. Review.
-
Experimental and Investigational _targeted Therapies for the Management of Fibrosis in NASH: An Update.J Exp Pharmacol. 2021 Mar 18;13:329-338. doi: 10.2147/JEP.S265286. eCollection 2021. J Exp Pharmacol. 2021. PMID: 33776490 Free PMC article. Review.
-
High-density lipoproteins and non-alcoholic fatty liver disease.Atheroscler Plus. 2023 Aug 19;53:33-41. doi: 10.1016/j.athplu.2023.08.001. eCollection 2023 Sep. Atheroscler Plus. 2023. PMID: 37663008 Free PMC article.
References
-
- Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL‐3196) for the treatment of non‐alcoholic steatohepatitis: a multicentre, randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet. 2019;394:2012‐2024. - PubMed
-
- Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator‐activated receptor‐alpha and ‐delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147‐1159.e1145. - PubMed
-
- Rinella ME, Trotter JF, Abdelmalek MF, et al. Rosuvastatin improves the FGF19 analogue NGM282‐associated lipid changes in patients with non‐alcoholic steatohepatitis. J Hepatol. 2019;70:735‐744. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

