Bcl-2 Overexpression and Hypoxia Synergistically Enhance Angiogenic Properties of Dental Pulp Stem Cells
- PMID: 32859045
- PMCID: PMC7503706
- DOI: 10.3390/ijms21176159
Bcl-2 Overexpression and Hypoxia Synergistically Enhance Angiogenic Properties of Dental Pulp Stem Cells
Abstract
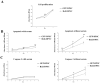
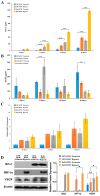
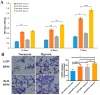
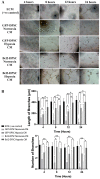
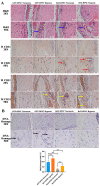
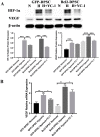
Post-implantation cell survival and angio-/vasculogenesis are critical for the success of cell-based regenerative strategies. The current study aimed to overexpress B-cell lymphoma 2 (Bcl-2) gene in dental pulp stem cells (DPSCs) and examine the anti-apoptotic and angio-/vasculogenic effects both in-vitro and in-vivo. DPSCs were transduced with Bcl-2-green fluorescent protein (GFP) lentiviral particles and examined for cell proliferation and apoptosis. The cells were cultured under normoxic or hypoxic (0.5 mM CoCl2) conditions and examined for the expression of angiogenic factors and effects on endothelial cell proliferation, migration and vessel morphogenesis. Cells with or without hypoxic preconditioning were used in in-vivo Matrigel plug assay to study the post-implantation cell survival and angio-/vasculogenesis. Bcl-2-overexpressing-DPSCs showed significantly lower apoptosis than that of null-GFP-DPSCs under serum-free conditions. Under hypoxia, Bcl-2-overexpressing-DPSCs expressed significantly higher levels of vascular endothelial growth factor compared to that under normoxia and null-GFP-DPSCs. Consequently, Bcl-2-overexpressing-DPSCs significantly enhanced endothelial cell proliferation, migration and vascular tube formation on Matrigel. Immunohistological assessment of in-vivo transplanted Matrigel plugs showed significantly higher cell survival and vasculature in hypoxic preconditioned Bcl-2-overexpressing-DPSC group compared to null-GFP-DPSC group. In conclusion, Bcl-2 overexpression and hypoxic-preconditioning could be synergistically used to enhance post-implantation cell survival and angio-/vasculogenic properties of DPSCs.
Keywords: Bcl-2; angiogenesis; dental pulp stem cells; gene modification; post-implantation cell survival; tissue regeneration; vascularization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration.Clin Oral Investig. 2019 May;23(5):2497-2509. doi: 10.1007/s00784-018-2699-0. Epub 2018 Oct 12. Clin Oral Investig. 2019. PMID: 30315421
-
Effects of Recombinant Overexpression of Bcl2 on the Proliferation, Apoptosis, and Osteogenic/Odontogenic Differentiation Potential of Dental Pulp Stem Cells.J Endod. 2016 Apr;42(4):575-83. doi: 10.1016/j.joen.2016.01.013. Epub 2016 Feb 18. J Endod. 2016. PMID: 26898562
-
VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells.J Dent Res. 2013 Jun;92(6):524-31. doi: 10.1177/0022034513485599. Epub 2013 Apr 22. J Dent Res. 2013. PMID: 23609159
-
Role of Hypoxia in Mesenchymal Stem Cells from Dental Pulp: Influence, Mechanism and Application.Cell Biochem Biophys. 2024 Jun;82(2):535-547. doi: 10.1007/s12013-024-01274-0. Epub 2024 May 7. Cell Biochem Biophys. 2024. PMID: 38713403 Free PMC article. Review.
-
Recycle the dental fairy's package: overview of dental pulp stem cells.Stem Cell Res Ther. 2018 Dec 13;9(1):347. doi: 10.1186/s13287-018-1094-8. Stem Cell Res Ther. 2018. PMID: 30545418 Free PMC article. Review.
Cited by
-
Modulation of the Dental Pulp Stem Cell Secretory Profile by Hypoxia Induction Using Cobalt Chloride.J Pers Med. 2021 Mar 30;11(4):247. doi: 10.3390/jpm11040247. J Pers Med. 2021. PMID: 33808091 Free PMC article.
-
Hypoxia Alters the Proteome Profile and Enhances the Angiogenic Potential of Dental Pulp Stem Cell-Derived Exosomes.Biomolecules. 2022 Apr 14;12(4):575. doi: 10.3390/biom12040575. Biomolecules. 2022. PMID: 35454164 Free PMC article.
-
Clinical Perspectives of Non-Coding RNA in Oral Inflammatory Diseases and Neuropathic Pain: A Narrative Review.Int J Mol Sci. 2022 Jul 27;23(15):8278. doi: 10.3390/ijms23158278. Int J Mol Sci. 2022. PMID: 35955417 Free PMC article. Review.
-
Metformin pre-conditioning enhances the angiogenic ability of the secretome of dental pulp stem cells.Saudi Pharm J. 2021 Aug;29(8):908-913. doi: 10.1016/j.jsps.2021.07.004. Epub 2021 Jul 16. Saudi Pharm J. 2021. PMID: 34408549 Free PMC article.
-
Prevascularization techniques for dental pulp regeneration: potential cell sources, intercellular communication and construction strategies.Front Bioeng Biotechnol. 2023 May 18;11:1186030. doi: 10.3389/fbioe.2023.1186030. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37274160 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

