Inhalational delivery of induced pluripotent stem cell secretome improves postpneumonectomy lung structure and function
- PMID: 32909918
- PMCID: PMC7790128
- DOI: 10.1152/japplphysiol.00205.2020
Inhalational delivery of induced pluripotent stem cell secretome improves postpneumonectomy lung structure and function
Abstract
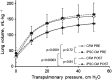
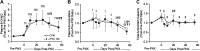
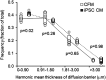
Cell-free secretory products (secretome) of human induced pluripotent stem cells (iPSCs) have been shown to attenuate tissue injury and facilitate repair and recovery. To examine whether iPSC secretome facilitates mechanically induced compensatory responses following unilateral pneumonectomy (PNX), litter-matched young adult female hounds underwent right PNX (removing 55%-58% of lung units), followed by inhalational delivery of either the nebulized-conditioned media containing induced pluripotent stem cell secretome (iPSC CM) or control cell-free media (CFM); inhalation was repeated every 5 days for 10 treatments. Lung function was measured under anesthesia pre-PNX and 10 days after the last treatment (8 wk post-PNX); detailed quantitative analysis of lung ultrastructure was performed postmortem. Pre-PNX lung function was similar between groups. Compared with CFM control, treatment with iPSC CM attenuated the post-PNX decline in lung diffusing capacity for carbon monoxide and membrane diffusing capacity, accompanied by a 24% larger postmortem lobar volume and distal air spaces. Alveolar double-capillary profiles were 39% more prevalent consistent with enhanced intussusceptive angiogenesis. Frequency distribution of the harmonic mean thickness of alveolar blood-gas barrier shifted toward the lowest values, whereas alveolar septal tissue volume and arithmetic septal thickness were similar, indicating septal remodeling and reduced diffusive resistance of the blood-gas barrier. Thus, repetitive inhalational delivery of iPSC secretome enhanced post-PNX alveolar angiogenesis and septal remodeling that are associated with improved gas exchange compensation. Results highlight the plasticity of the remaining lung units following major loss of lung mass that are responsive to broad-based modulation provided by the iPSC secretome.NEW & NOTEWORTHY To examine whether the secreted products of human induced pluripotent stem cells (iPSCs) facilitate innate adaptive responses following loss of lung tissue, adult dogs underwent surgical removal of one lung, then received repeated administration of iPSC secretory products via inhalational delivery compared with control treatment. Inhalation of iPSC secretory products enhanced capillary formation and beneficial structural remodeling in the remaining lung, leading to improved lung function.
Keywords: alveolar remodeling; compensatory lung growth; induced pluripotent stem cells; lung diffusing capacity; secretome.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Erythropoietin inhalation enhances adult canine alveolar-capillary formation following pneumonectomy.Am J Physiol Lung Cell Mol Physiol. 2019 May 1;316(5):L936-L945. doi: 10.1152/ajplung.00504.2018. Epub 2019 Feb 20. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30785346 Free PMC article.
-
In vivo imaging of canine lung deformation: effects of posture, pneumonectomy, and inhaled erythropoietin.J Appl Physiol (1985). 2020 May 1;128(5):1093-1105. doi: 10.1152/japplphysiol.00647.2019. Epub 2020 Jan 16. J Appl Physiol (1985). 2020. PMID: 31944885 Free PMC article.
-
Long-term post-pneumonectomy pulmonary adaptation following all-trans-retinoic acid supplementation.J Appl Physiol (1985). 2011 Mar;110(3):764-73. doi: 10.1152/japplphysiol.00994.2010. Epub 2010 Nov 25. J Appl Physiol (1985). 2011. PMID: 21109601 Free PMC article.
-
Further examination of alveolar septal adaptation to left pneumonectomy in the adult lung.Respir Physiol Neurobiol. 2006 Apr 28;151(2-3):167-77. doi: 10.1016/j.resp.2006.01.013. Epub 2006 Mar 24. Respir Physiol Neurobiol. 2006. PMID: 16563882 Review.
-
The pneumonectomy model of compensatory lung growth: insights into lung regeneration.Pharmacol Ther. 2014 May;142(2):196-205. doi: 10.1016/j.pharmthera.2013.12.006. Epub 2013 Dec 12. Pharmacol Ther. 2014. PMID: 24333263 Review.
Cited by
-
Stem Cell's Secretome Delivery Systems.Adv Pharm Bull. 2023 Mar;13(2):244-258. doi: 10.34172/apb.2023.027. Epub 2022 Jan 3. Adv Pharm Bull. 2023. PMID: 37342369 Free PMC article. Review.
-
Translational Animal Models Provide Insight Into Mesenchymal Stromal Cell (MSC) Secretome Therapy.Front Cell Dev Biol. 2021 Mar 19;9:654885. doi: 10.3389/fcell.2021.654885. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869217 Free PMC article. Review.
-
Extracellular vesicle-mediated cellular crosstalk in lung repair, remodelling and regeneration.Eur Respir Rev. 2022 Jan 25;31(163):210106. doi: 10.1183/16000617.0106-2021. Print 2022 Mar 31. Eur Respir Rev. 2022. PMID: 35082125 Free PMC article. Review.
References
-
- Asmussen S, Ito H, Traber DL, Lee JW, Cox RA, Hawkins HK, McAuley DF, McKenna DH, Traber LD, Zhuo H, Wilson J, Herndon DN, Prough DS, Liu KD, Matthay MA, Enkhbaatar P. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax 69: 819–825, 2014. doi:10.1136/thoraxjnl-2013-204980. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

