Single oral fixed-dose praziquantel-miltefosine nanocombination for effective control of experimental schistosomiasis mansoni
- PMID: 32933556
- PMCID: PMC7493353
- DOI: 10.1186/s13071-020-04346-1
Single oral fixed-dose praziquantel-miltefosine nanocombination for effective control of experimental schistosomiasis mansoni
Abstract
Background: The control of schistosomiasis has been centered to date on a single drug, praziquantel, with shortcomings including treatment failure, reinfection, and emergence of drug resistance. Drug repurposing, combination therapy or nanotechnology were explored to improve antischistosomal treatment. The aim of the present study was to utilize a novel combination of the three strategies to improve the therapeutic profile of praziquantel. This was based on a fixed-dose nanocombination of praziquantel and miltefosine, an antischistosomal repurposing candidate, co-loaded at reduced doses into lipid nanocapsules, for single dose oral therapy.
Methods: Two nanocombinations were prepared to provide 250 mg praziquantel-20 mg miltefosine/kg (higher fixed-dose) or 125 mg praziquantel-10 mg miltefosine/kg (lower fixed-dose), respectively. Their antischistosomal efficacy in comparison with a non-treated control and their praziquantel or miltefosine singly loaded counterparts was assessed in murine schistosomiasis mansoni. A single oral dose of either formulation was administered on the initial day of infection, and on days 21 and 42 post-infection. Scanning electron microscopic, parasitological, and histopathological studies were used for assessment. Preclinical data were subjected to analysis of variance and Tukey's post-hoc test for pairwise comparisons.
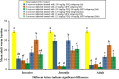
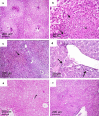
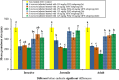
Results: Lipid nanocapsules (~ 58 nm) showed high entrapment efficiency of both drugs (> 97%). Compared to singly loaded praziquantel-lipid nanocapsules, the higher nanocombination dose showed a significant increase in antischistosomal efficacy in terms of statistically significant decrease in mean worm burden, particularly against invasive and juvenile worms, and amelioration of hepatic granulomas (P ≤ 0.05). In addition, scanning electron microscopy examination showed extensive dorsal tegumental damage with noticeable deposition of nanostructures.
Conclusions: The therapeutic profile of praziquantel could be improved by a novel multiple approach integrating drug repurposing, combination therapy and nanotechnology. Multistage activity and amelioration of liver pathology could be achieved by a new praziquantel-miltefosine fixed-dose nanocombination providing 250 mg praziquantel-20 mg miltefosine/kg. To the best of our knowledge, this is the first report of a fixed-dose nano-based combinatorial therapy for schistosomiasis mansoni. Further studies are needed to document the nanocombination safety and explore its prophylactic activity and potential to hinder the onset of resistance to the drug components.
Keywords: Lipid Nanocapsules; Miltefosine; Multistage activity; Nanocombination; Praziquantel; Scanning electron microscopy; Schistosoma mansoni; Tegumental _targeting.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Evaluation of prophylactic efficacy and safety of praziquantel-miltefosine nanocombination in experimental Schistosomiasis mansoni.Acta Trop. 2020 Dec;212:105714. doi: 10.1016/j.actatropica.2020.105714. Epub 2020 Sep 17. Acta Trop. 2020. PMID: 32950482
-
Miltefosine lipid nanocapsules: Intersection of drug repurposing and nanotechnology for single dose oral treatment of pre-patent schistosomiasis mansoni.Acta Trop. 2016 Jul;159:142-8. doi: 10.1016/j.actatropica.2016.03.038. Epub 2016 Mar 31. Acta Trop. 2016. PMID: 27039667
-
Miltefosine Lipid Nanocapsules for Single Dose Oral Treatment of Schistosomiasis Mansoni: A Preclinical Study.PLoS One. 2015 Nov 17;10(11):e0141788. doi: 10.1371/journal.pone.0141788. eCollection 2015. PLoS One. 2015. PMID: 26574746 Free PMC article.
-
Efficacy of praziquantel and artemisinin derivatives for the treatment and prevention of human schistosomiasis: a systematic review and meta-analysis.Parasit Vectors. 2011 Oct 17;4:201. doi: 10.1186/1756-3305-4-201. Parasit Vectors. 2011. PMID: 22004571 Free PMC article. Review.
-
Improving translational power in antischistosomal drug discovery.Adv Parasitol. 2022;117:47-73. doi: 10.1016/bs.apar.2022.05.002. Epub 2022 Jun 29. Adv Parasitol. 2022. PMID: 35878949 Review.
Cited by
-
Appraisal of Chitosan-Coated Lipid Nano-Combination with Miltefosine and Albendazole in the Treatment of Murine Trichinellosis: Experimental Study with Evaluation of Immunological and Immunohistochemical Parameters.Acta Parasitol. 2024 Mar;69(1):929-950. doi: 10.1007/s11686-024-00799-x. Epub 2024 Mar 15. Acta Parasitol. 2024. PMID: 38489009 Free PMC article.
-
Nanotechnological approaches in the treatment of schistosomiasis: an overview.Beilstein J Nanotechnol. 2024 Jan 3;15:13-25. doi: 10.3762/bjnano.15.2. eCollection 2024. Beilstein J Nanotechnol. 2024. PMID: 38213572 Free PMC article. Review.
-
Enhancing the in vitro and in vivo activity of itraconazole against breast cancer using miltefosine-modified lipid nanocapsules.Drug Deliv. 2021 Dec;28(1):906-919. doi: 10.1080/10717544.2021.1917728. Drug Deliv. 2021. PMID: 33960245 Free PMC article.
-
Potential application of nanotechnology in the treatment, diagnosis, and prevention of schistosomiasis.Front Bioeng Biotechnol. 2022 Dec 9;10:1013354. doi: 10.3389/fbioe.2022.1013354. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36568300 Free PMC article. Review.
-
Tunable Polymeric Mixed Micellar Nanoassemblies of Lutrol F127/Gelucire 44/14 for Oral Delivery of Praziquantel: A Promising Nanovector against Hymenolepis nana in Experimentally-Infected Rats.Pharmaceutics. 2022 Sep 23;14(10):2023. doi: 10.3390/pharmaceutics14102023. Pharmaceutics. 2022. PMID: 36297459 Free PMC article.
References
-
- LoVerde PT. Schistosomiasis. In: Toledo R, Fried B, editors. Digenetic trematodes. Cham: Springer International Publishing; 2019. pp. 45–70.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

