Astrocytic glutamate transporter 1 (GLT1) deficient mice exhibit repetitive behaviors
- PMID: 32950606
- PMCID: PMC7572885
- DOI: 10.1016/j.bbr.2020.112906
Astrocytic glutamate transporter 1 (GLT1) deficient mice exhibit repetitive behaviors
Erratum in
-
Corrigendum to "Astrocytic glutamate transporter 1 (GLT1) deficient mice exhibit repetitive behaviors" [Behav. Brain Res. 396 (2021) 112906].Behav Brain Res. 2022 Mar 26;422:113749. doi: 10.1016/j.bbr.2022.113749. Epub 2022 Jan 19. Behav Brain Res. 2022. PMID: 35065422 No abstract available.
Abstract
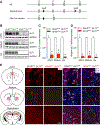
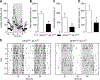
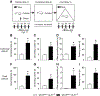
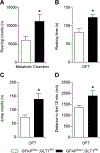
Glutamatergic dysregulation is known to contribute to obsessive-compulsive disorder (OCD). Astrocytic glutamate transporter 1 (GLT1) is responsible for the majority of glutamate clearance. However, the role of GLT1 in OCD-like behavior remains unclear. Here, we found that astrocytic GLT1 deficient mice showed increased wheel running activity but reduced home cage activity. Notably, they exhibited elevated grooming/rearing time and increased repetitive behavior counts in contextual and cued fear conditioning. In addition, they showed increased rearing counts in the metabolic chamber, and also augmented rearing time and jumping counts in the open field test. Taken together, our findings suggest that astrocytic GLT1 deficiency promotes OCD-like repetitive behaviors.
Keywords: Fear conditioning; Glutamate transporter 1 (GLT1); Grooming; Obsessive-compulsive disorder (OCD); Repetitive behavior; Wheel running activity.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Disclosures
Dr. DS Choi is a scientific advisory board member to Peptron Inc. and the Peptron had no role in the preparation, review, or approval of the manuscript; nor the decision to submit the manuscript for publication. All the other authors declare no biomedical financial interests or potential conflicts of interest.
Figures
Similar articles
-
Astrocytic Glutamate Transporter 1 (GLT1) Deficiency Reduces Anxiety- and Depression-Like Behaviors in Mice.Front Behav Neurosci. 2020 Apr 22;14:57. doi: 10.3389/fnbeh.2020.00057. eCollection 2020. Front Behav Neurosci. 2020. PMID: 32390810 Free PMC article.
-
Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice.Neuropsychopharmacology. 2015 Jun;40(7):1569-79. doi: 10.1038/npp.2015.26. Epub 2015 Feb 9. Neuropsychopharmacology. 2015. PMID: 25662838 Free PMC article.
-
Experience-dependent grooming microstructure alterations and gastrointestinal dysfunction in the SAPAP3 knockout mouse model of compulsive behaviour.J Affect Disord. 2024 Oct 15;363:520-531. doi: 10.1016/j.jad.2024.07.143. Epub 2024 Jul 21. J Affect Disord. 2024. PMID: 39043310
-
Molecular and cellular basis of obsessive-compulsive disorder-like behaviors: emerging view from mouse models.Curr Opin Neurol. 2011 Apr;24(2):114-8. doi: 10.1097/WCO.0b013e32834451fb. Curr Opin Neurol. 2011. PMID: 21386675 Review.
-
A review of animal models of obsessive-compulsive disorder: a focus on developmental, immune, endocrine and behavioral models.Expert Opin Drug Discov. 2016;11(1):27-43. doi: 10.1517/17460441.2016.1103225. Epub 2015 Nov 11. Expert Opin Drug Discov. 2016. PMID: 26558411 Review.
Cited by
-
Rethinking the Approach to Preclinical Models of Anorexia Nervosa.Curr Psychiatry Rep. 2022 Jan;24(1):71-76. doi: 10.1007/s11920-022-01319-2. Epub 2022 Feb 11. Curr Psychiatry Rep. 2022. PMID: 35147866 Free PMC article. Review.
-
The role of glutamate and glutamine metabolism and related transporters in nerve cells.CNS Neurosci Ther. 2024 Feb;30(2):e14617. doi: 10.1111/cns.14617. CNS Neurosci Ther. 2024. PMID: 38358002 Free PMC article. Review.
-
Memantine/Aripiprazole Combination Alleviates Cognitive Dysfunction in Valproic Acid Rat Model of Autism: Hippocampal CREB/BDNF Signaling and Glutamate Homeostasis.Neurotherapeutics. 2023 Mar;20(2):464-483. doi: 10.1007/s13311-023-01360-w. Epub 2023 Mar 14. Neurotherapeutics. 2023. PMID: 36918475 Free PMC article.
-
Body Focused Repetitive Behavior Disorders: Behavioral Models and Neurobiological Mechanisms.Turk Psikiyatri Derg. 2023 Spring;34(1):50-59. doi: 10.5080/u26213. Turk Psikiyatri Derg. 2023. PMID: 36970962 Free PMC article. Review.
-
Multi-omic profiling of the developing human cerebral cortex at the single-cell level.Sci Adv. 2023 Oct 13;9(41):eadg3754. doi: 10.1126/sciadv.adg3754. Epub 2023 Oct 12. Sci Adv. 2023. PMID: 37824614 Free PMC article.
References
-
- Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S, Glutamatergic dysfunction in OCD, Neuropsychopharmacology 30(9) (2005) 1735–40. - PubMed
-
- McGrath MJ, Campbell KM, Parks CR, Burton FH, Glutamatergic drugs exacerbate symptomatic behavior in a transgenic model of comorbid Tourette’s syndrome and obsessive-compulsive disorder, Brain research 877(1) (2000) 23–30. - PubMed
-
- Moore GJ, MacMaster FP, Stewart C, Rosenberg DR, Case study: caudate glutamatergic changes with paroxetine therapy for pediatric obsessive-compulsive disorder, Journal of the American Academy of Child and Adolescent Psychiatry 37(6) (1998) 663–7. - PubMed
-
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ, Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine, Journal of the American Academy of Child and Adolescent Psychiatry 39(9) (2000) 1096–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

