Brca2 deficiency drives gastrointestinal tumor formation and is selectively inhibited by mitomycin C
- PMID: 32980867
- PMCID: PMC7519908
- DOI: 10.1038/s41419-020-03013-8
Brca2 deficiency drives gastrointestinal tumor formation and is selectively inhibited by mitomycin C
Abstract
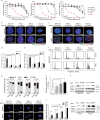
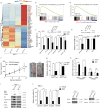
BRCA2 is crucial for repairing DNA double-strand breaks with high fidelity, and loss of BRCA2 increases the risks of developing breast and ovarian cancers. Herein, we show that BRCA2 is inactively mutated in 10% of gastric and 7% of colorectal adenocarcinomas, and that this inactivation is significantly correlated with microsatellite instability. Villin-driven Brca2 depletion promotes mouse gastrointestinal tumor formation when genome instability is increased. Whole-genome screening data showed that these BRCA2 monoallelic and biallelic mutant tumors were selectively inhibited by mitomycin C. Mechanistically, mitomycin C provoked double-strand breaks in cancer cells that often recruit wild-type BRCA2 for repair; the failure to repair double-strand breaks caused cell-cycle arrest at the S phase and p53-mediated cell apoptosis of BRCA2 monoallelic and biallelic mutant tumor cells. Our study unveils the role of BRCA2 loss in the development of gastrointestinal tumors and provides a potential therapeutic strategy to eliminate BRCA2 monoallelic and biallelic mutant tumors through mitomycin C.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Does tumorigenesis select for or against mutations of the DNA repair-associated genes BRCA2 and MRE11?: considerations from somatic mutations in microsatellite unstable (MSI) gastrointestinal cancers.BMC Genet. 2006 Jan 17;7:3. doi: 10.1186/1471-2156-7-3. BMC Genet. 2006. PMID: 16417627 Free PMC article.
-
Lower level of BRCA2 protein in heterozygous mutation carriers is correlated with an increase in DNA double strand breaks and an impaired DSB repair.Cancer Lett. 2006 Nov 8;243(1):90-100. doi: 10.1016/j.canlet.2005.11.041. Epub 2006 Jan 31. Cancer Lett. 2006. PMID: 16448746
-
Loss of Brca2 and p53 synergistically promotes genomic instability and deregulation of T-cell apoptosis.Cancer Res. 2002 Nov 1;62(21):6194-204. Cancer Res. 2002. PMID: 12414647
-
A comprehensive analysis of BRCA2 gene: focus on mechanistic aspects of its functions, spectrum of deleterious mutations, and therapeutic strategies _targeting BRCA2-deficient tumors.Med Oncol. 2018 Jan 31;35(3):18. doi: 10.1007/s12032-018-1085-8. Med Oncol. 2018. PMID: 29387975 Review.
-
Therapeutic exploitation of tumor cell defects in homologous recombination.Anticancer Agents Med Chem. 2008 May;8(4):448-60. doi: 10.2174/187152008784220267. Anticancer Agents Med Chem. 2008. PMID: 18473729 Review.
Cited by
-
Mitomycin C in Homologous Recombination Deficient Metastatic Pancreatic Cancer after Disease Progression on Platinum-Based Chemotherapy and Olaparib.Biomedicines. 2022 Oct 26;10(11):2705. doi: 10.3390/biomedicines10112705. Biomedicines. 2022. PMID: 36359225 Free PMC article.
-
Thioparib inhibits homologous recombination repair, activates the type I IFN response, and overcomes olaparib resistance.EMBO Mol Med. 2023 Mar 8;15(3):e16235. doi: 10.15252/emmm.202216235. Epub 2023 Jan 18. EMBO Mol Med. 2023. PMID: 36652375 Free PMC article.
-
Expression of Four Autophagy-Related Genes Accurately Predicts the Prognosis of Gastrointestinal Cancer in Asian Patients.Dis Markers. 2021 Aug 25;2021:7253633. doi: 10.1155/2021/7253633. eCollection 2021. Dis Markers. 2021. PMID: 34484469 Free PMC article.
References
-
- Wong AK, Pero R, Ormonde PA, Tavtigian SV, Bartel PL. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene brca2. J. Biol. Chem. 1997;272:31941–31944. - PubMed
-
- Davies AA, et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol. Cell. 2001;7:273–282. - PubMed
-
- Patel KJ, et al. Involvement of Brca2 in DNA repair. Mol. Cell. 1998;1:347–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

