Structural Characterization of a Novel Two-Dimensional Material: Cobalt Sulfide Sheets on Au(111)
- PMID: 32986432
- PMCID: PMC7649848
- DOI: 10.1021/acs.jpclett.0c02268
Structural Characterization of a Novel Two-Dimensional Material: Cobalt Sulfide Sheets on Au(111)
Abstract
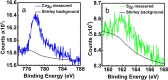
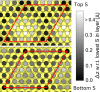
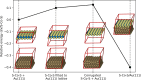
Transition metal dichalcogenides (TMDCs) are a type of two-dimensional (2D) material that has been widely investigated by both experimentalists and theoreticians because of their unique properties. In the case of cobalt sulfide, density functional theory (DFT) calculations on free-standing S-Co-S sheets suggest there are no stable 2D cobalt sulfide polymorphs, whereas experimental observations clearly show TMDC-like structures on Au(111). In this study, we resolve this disagreement by using a combination of experimental techniques and DFT calculations, considering the substrate explicitly. We find a 2D CoS(0001)-like sheet on Au(111) that delivers excellent agreement between theory and experiment. Uniquely this sheet exhibits a metallic character, contrary to most TMDCs, and exists due to the stabilizing interactions with the Au(111) substrate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Reconstruction of Gold Surface with Excessive Sulfur Source During Transition Metal Disulfide Growth.Precis Chem. 2024 Jun 5;2(8):414-420. doi: 10.1021/prechem.4c00018. eCollection 2024 Aug 26. Precis Chem. 2024. PMID: 39474151 Free PMC article.
-
Size-dependent phase stability in transition metal dichalcogenide nanoparticles controlled by metal substrates.Nanoscale. 2021 Jun 14;13(22):10167-10180. doi: 10.1039/d0nr08598g. Epub 2021 Jun 1. Nanoscale. 2021. PMID: 34075922
-
Predicting Single-Layer Technetium Dichalcogenides (TcX₂, X = S, Se) with Promising Applications in Photovoltaics and Photocatalysis.ACS Appl Mater Interfaces. 2016 Mar 2;8(8):5385-92. doi: 10.1021/acsami.5b12606. Epub 2016 Feb 18. ACS Appl Mater Interfaces. 2016. PMID: 26859697
-
Two-dimensional transition metal dichalcogenides: interface and defect engineering.Chem Soc Rev. 2018 May 8;47(9):3100-3128. doi: 10.1039/c8cs00024g. Chem Soc Rev. 2018. PMID: 29509206 Review.
-
Two-dimensional inorganic analogues of graphene: transition metal dichalcogenides.Philos Trans A Math Phys Eng Sci. 2016 Sep 13;374(2076):20150318. doi: 10.1098/rsta.2015.0318. Philos Trans A Math Phys Eng Sci. 2016. PMID: 27501969 Free PMC article. Review.
Cited by
-
Hydrodesulfurization of methanethiol over Co-promoted MoS2 model catalysts.Nat Commun. 2024 Aug 21;15(1):7170. doi: 10.1038/s41467-024-51549-6. Nat Commun. 2024. PMID: 39169026 Free PMC article.
References
-
- Cao X. H.; Yin Z. Y.; Zhang H. Three-dimensional Graphene Materials: Preparation, Structures and Application in Supercapacitors. Energy Environ. Sci. 2014, 7, 1850–1865. 10.1039/C4EE00050A. - DOI
LinkOut - more resources
Full Text Sources

