SARS-CoV-2 Viral Load Predicts Mortality in Patients with and without Cancer Who Are Hospitalized with COVID-19
- PMID: 32997958
- PMCID: PMC7492074
- DOI: 10.1016/j.ccell.2020.09.007
SARS-CoV-2 Viral Load Predicts Mortality in Patients with and without Cancer Who Are Hospitalized with COVID-19
Abstract
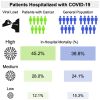
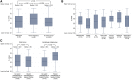
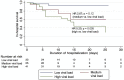
Patients with cancer may be at increased risk of severe coronavirus disease 2019 (COVID-19), but the role of viral load on this risk is unknown. We measured SARS-CoV-2 viral load using cycle threshold (CT) values from reverse-transcription polymerase chain reaction assays applied to nasopharyngeal swab specimens in 100 patients with cancer and 2,914 without cancer who were admitted to three New York City hospitals. Overall, the in-hospital mortality rate was 38.8% among patients with a high viral load, 24.1% among patients with a medium viral load, and 15.3% among patients with a low viral load (p < 0.001). Similar findings were observed in patients with cancer (high, 45.2% mortality; medium, 28.0%; low, 12.1%; p = 0.008). Patients with hematologic malignancies had higher median viral loads (CT = 25.0) than patients without cancer (CT = 29.2; p = 0.0039). SARS-CoV-2 viral load results may offer vital prognostic information for patients with and without cancer who are hospitalized with COVID-19.
Keywords: cancer; coronavirus disease 2019 (COVID-19); cycle threshold (C(T)); hematologic malignancy; mortality; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); solid tumor; viral load.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests L.F.W. reports receiving consulting and grant support from Roche Molecular Systems, Inc. M.M.S. receives grant support from Amgen, Inc. All other authors declare no competing interests.
Figures

Similar articles
-
Covid-19 in patients with hematological and solid cancers at a Comprehensive Cancer Center in Germany.Cancer Med. 2020 Nov;9(22):8412-8422. doi: 10.1002/cam4.3460. Epub 2020 Sep 15. Cancer Med. 2020. PMID: 32931637 Free PMC article.
-
Convalescent Plasma for the Treatment of Severe COVID-19 Infection in Cancer Patients.Cancer Med. 2020 Nov;9(22):8571-8578. doi: 10.1002/cam4.3457. Epub 2020 Sep 17. Cancer Med. 2020. PMID: 32945149 Free PMC article.
-
High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR.Eur J Cancer. 2020 Aug;135:251-259. doi: 10.1016/j.ejca.2020.05.028. Epub 2020 Jun 7. Eur J Cancer. 2020. PMID: 32540204 Free PMC article.
-
Why COVID-19 Transmission Is More Efficient and Aggressive Than Viral Transmission in Previous Coronavirus Epidemics?Biomolecules. 2020 Sep 11;10(9):1312. doi: 10.3390/biom10091312. Biomolecules. 2020. PMID: 32933047 Free PMC article. Review.
-
Inferred duration of infectious period of SARS-CoV-2: rapid scoping review and analysis of available evidence for asymptomatic and symptomatic COVID-19 cases.BMJ Open. 2020 Aug 5;10(8):e039856. doi: 10.1136/bmjopen-2020-039856. BMJ Open. 2020. PMID: 32759252 Free PMC article. Review.
Cited by
-
SARS-CoV-2 viral load is linked to remdesivir efficacy in severe Covid-19 admitted to intensive care.Sci Rep. 2024 Sep 6;14(1):20825. doi: 10.1038/s41598-024-71588-9. Sci Rep. 2024. PMID: 39242658 Free PMC article.
-
Neutralizing monoclonal antibodies for treatment of COVID-19.Nat Rev Immunol. 2021 Jun;21(6):382-393. doi: 10.1038/s41577-021-00542-x. Epub 2021 Apr 19. Nat Rev Immunol. 2021. PMID: 33875867 Free PMC article. Review.
-
Isolation of SARS-CoV-2 in Viral Cell Culture in Immunocompromised Patients With Persistently Positive RT-PCR Results.Front Cell Infect Microbiol. 2022 Feb 2;12:804175. doi: 10.3389/fcimb.2022.804175. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35186791 Free PMC article.
-
The presence of SARS-CoV-2 in multiple clinical specimens of a fatal case of COVID-19: a case report.J Med Case Rep. 2022 Dec 22;16(1):484. doi: 10.1186/s13256-022-03706-y. J Med Case Rep. 2022. PMID: 36550575 Free PMC article.
-
Clinical Characteristics, Management, and Outcomes of Cancer Patients With Coronavirus Disease 2019 Admitted to the ICU.Crit Care Explor. 2021 Sep 7;3(9):e0535. doi: 10.1097/CCE.0000000000000535. eCollection 2021 Sep. Crit Care Explor. 2021. PMID: 34514429 Free PMC article.
References
-
- cobas cobas SARS-CoV-2 Assay Instructions for Use v1.0. 2020. https://www.fda.gov/media/136049/download
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

