REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters
- PMID: 33037066
- PMCID: PMC7857396
- DOI: 10.1126/science.abe2402
REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters
Abstract
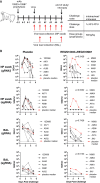
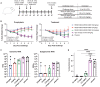
An urgent global quest for effective therapies to prevent and treat coronavirus disease 2019 (COVID-19) is ongoing. We previously described REGN-COV2, a cocktail of two potent neutralizing antibodies (REGN10987 and REGN10933) that _targets nonoverlapping epitopes on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein. In this report, we evaluate the in vivo efficacy of this antibody cocktail in both rhesus macaques, which may model mild disease, and golden hamsters, which may model more severe disease. We demonstrate that REGN-COV-2 can greatly reduce virus load in the lower and upper airways and decrease virus-induced pathological sequelae when administered prophylactically or therapeutically in rhesus macaques. Similarly, administration in hamsters limits weight loss and decreases lung titers and evidence of pneumonia in the lungs. Our results provide evidence of the therapeutic potential of this antibody cocktail.
Copyright © 2020, American Association for the Advancement of Science.
Figures

Similar articles
-
REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19.N Engl J Med. 2021 Jan 21;384(3):238-251. doi: 10.1056/NEJMoa2035002. Epub 2020 Dec 17. N Engl J Med. 2021. PMID: 33332778 Free PMC article. Clinical Trial.
-
REGN-COV2 antibody cocktail in patients with SARS-CoV-2: Observational study from a single institution in Japan.J Infect Chemother. 2022 Jul;28(7):943-947. doi: 10.1016/j.jiac.2022.03.029. Epub 2022 Apr 7. J Infect Chemother. 2022. PMID: 35414436 Free PMC article.
-
Neutralizing Monoclonal Antibodies That _target the Spike Receptor Binding Domain Confer Fc Receptor-Independent Protection against SARS-CoV-2 Infection in Syrian Hamsters.mBio. 2021 Oct 26;12(5):e0239521. doi: 10.1128/mBio.02395-21. Epub 2021 Sep 14. mBio. 2021. PMID: 34517754 Free PMC article.
-
Melatonin and REGN-CoV2 combination as a vaccine adjuvant for Omicron variant of SARS-CoV-2.Mol Biol Rep. 2022 May;49(5):4061-4068. doi: 10.1007/s11033-022-07419-9. Epub 2022 Apr 7. Mol Biol Rep. 2022. PMID: 35389130 Free PMC article. Review.
-
Neutralizing antibodies against SARS-CoV-2: current understanding, challenge and perspective.Antib Ther. 2020 Dec 28;3(4):285-299. doi: 10.1093/abt/tbaa028. eCollection 2020 Dec. Antib Ther. 2020. PMID: 33912797 Free PMC article. Review.
Cited by
-
Success of prophylactic antiviral therapy for SARS-CoV-2: Predicted critical efficacies and impact of different drug-specific mechanisms of action.PLoS Comput Biol. 2021 Mar 1;17(3):e1008752. doi: 10.1371/journal.pcbi.1008752. eCollection 2021 Mar. PLoS Comput Biol. 2021. PMID: 33647008 Free PMC article.
-
Neutralizing Antibody Therapeutics for COVID-19.Viruses. 2021 Apr 7;13(4):628. doi: 10.3390/v13040628. Viruses. 2021. PMID: 33916927 Free PMC article. Review.
-
Decay of Fc-dependent antibody functions after mild to moderate COVID-19.Cell Rep Med. 2021 Jun 15;2(6):100296. doi: 10.1016/j.xcrm.2021.100296. Epub 2021 May 9. Cell Rep Med. 2021. PMID: 33997824 Free PMC article.
-
The temperature-dependent conformational ensemble of SARS-CoV-2 main protease (Mpro).IUCrJ. 2022 Aug 17;9(Pt 5):682-694. doi: 10.1107/S2052252522007497. eCollection 2022 Sep 1. IUCrJ. 2022. PMID: 36071812 Free PMC article.
-
Identification of hACE2-interacting sites in SARS-CoV-2 spike receptor binding domain for antiviral drugs screening.Virus Res. 2022 Nov;321:198915. doi: 10.1016/j.virusres.2022.198915. Epub 2022 Sep 6. Virus Res. 2022. PMID: 36084746 Free PMC article.
References
-
- Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S., Tang X., Yu J., Lan J., Yuan J., Wang H., Zhao J., Zhang S., Wang Y., Shi X., Liu L., Zhao J., Wang X., Zhang Z., Zhang L., Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 584, 115–119 (2020). 10.1038/s41586-020-2380-z - DOI - PubMed
-
- Pinto D., Park Y.-J., Beltramello M., Walls A. C., Tortorici M. A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., Peter A., Guarino B., Spreafico R., Cameroni E., Case J. B., Chen R. E., Havenar-Daughton C., Snell G., Telenti A., Virgin H. W., Lanzavecchia A., Diamond M. S., Fink K., Veesler D., Corti D., Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295 (2020). 10.1038/s41586-020-2349-y - DOI - PubMed
-
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C., Chai X., He R., Li X., Lv Q., Zhu H., Deng W., Xu Y., Wang Y., Qiao L., Tan Y., Song L., Wang G., Du X., Gao N., Liu J., Xiao J., Su X. D., Du Z., Feng Y., Qin C., Qin C., Jin R., Xie X. S., Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 182, 73–84.e16 (2020). 10.1016/j.cell.2020.05.025 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

