The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-tumor Activity
- PMID: 33117715
- PMCID: PMC7575731
- DOI: 10.3389/fonc.2020.578418
The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-tumor Activity
Abstract
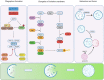
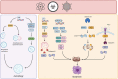
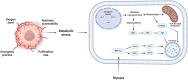
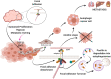
During tumorigenesis, cancer cells are exposed to a wide variety of intrinsic and extrinsic stresses that challenge homeostasis and growth. Cancer cells display activation of distinct mechanisms for adaptation and growth even in the presence of stress. Autophagy is a catabolic mechanism that aides in the degradation of damaged intracellular material and metabolite recycling. This activity helps meet metabolic needs during nutrient deprivation, genotoxic stress, growth factor withdrawal and hypoxia. However, autophagy plays a paradoxical role in tumorigenesis, depending on the stage of tumor development. Early in tumorigenesis, autophagy is a tumor suppressor via degradation of potentially oncogenic molecules. However, in advanced stages, autophagy promotes the survival of tumor cells by ameliorating stress in the microenvironment. These roles of autophagy are intricate due to their interconnection with other distinct cellular pathways. In this review, we present a broad view of the participation of autophagy in distinct phases of tumor development. Moreover, autophagy participation in important cellular processes such as cell death, metabolic reprogramming, metastasis, immune evasion and treatment resistance that all contribute to tumor development, is reviewed. Finally, the contribution of the hypoxic and nutrient deficient tumor microenvironment in regulation of autophagy and these hallmarks for the development of more aggressive tumors is discussed.
Keywords: autophagy; carcinogenesis; cell death; chemotherapy and _targeted therapy resistance; immune evasion; metabolic reprograming; metastasis; tumor microenvironment.
Copyright © 2020 Chavez-Dominguez, Perez-Medina, Lopez-Gonzalez, Galicia-Velasco and Aguilar-Cazares.
Figures
Similar articles
-
Delineating the twin role of autophagy in lung cancer.Biol Futur. 2023 Jun;74(1-2):119-135. doi: 10.1007/s42977-023-00165-4. Epub 2023 Apr 30. Biol Futur. 2023. PMID: 37120768 Review.
-
The Multifaceted Effects of Autophagy on the Tumor Microenvironment.Adv Exp Med Biol. 2020;1225:99-114. doi: 10.1007/978-3-030-35727-6_7. Adv Exp Med Biol. 2020. PMID: 32030650 Review.
-
Crosstalk Between ROS and Autophagy in Tumorigenesis: Understanding the Multifaceted Paradox.Front Oncol. 2022 Mar 10;12:852424. doi: 10.3389/fonc.2022.852424. eCollection 2022. Front Oncol. 2022. PMID: 35359388 Free PMC article. Review.
-
Molecular Mechanisms of Autophagy in Cancer Development, Progression, and Therapy.Biomedicines. 2022 Jul 5;10(7):1596. doi: 10.3390/biomedicines10071596. Biomedicines. 2022. PMID: 35884904 Free PMC article. Review.
-
Autophagy in the crosstalk between tumor and microenvironment.Cancer Lett. 2020 Oct 10;490:143-153. doi: 10.1016/j.canlet.2020.06.015. Epub 2020 Jul 4. Cancer Lett. 2020. PMID: 32634449 Review.
Cited by
-
Mesoporous Silica Nanoparticles Enhance the Anticancer Efficacy of Platinum(IV)-Phenolate Conjugates in Breast Cancer Cell Lines.Nanomaterials (Basel). 2022 Oct 26;12(21):3767. doi: 10.3390/nano12213767. Nanomaterials (Basel). 2022. PMID: 36364539 Free PMC article.
-
Investigation and confirmation of differentially expressed miRNAs, as well as _target gene prediction in papillary thyroid cancer, with a special emphasis on the autophagy signaling pathway.Mol Biol Res Commun. 2022;11(4):173-181. doi: 10.22099/mbrc.2022.43844.1751. Mol Biol Res Commun. 2022. PMID: 36777002 Free PMC article.
-
EGR1 Regulation of Vasculogenic Mimicry in the MDA-MB-231 Triple-Negative Breast Cancer Cell Line through the Upregulation of KLF4 Expression.Int J Mol Sci. 2023 Sep 21;24(18):14375. doi: 10.3390/ijms241814375. Int J Mol Sci. 2023. PMID: 37762678 Free PMC article.
-
The related miRNAs involved in doxorubicin resistance or sensitivity of various cancers: an update.Cancer Chemother Pharmacol. 2021 Nov;88(5):771-793. doi: 10.1007/s00280-021-04337-8. Epub 2021 Sep 12. Cancer Chemother Pharmacol. 2021. PMID: 34510251 Review.
-
Marine Cyanobacterial Peptides in Neuroblastoma: Search for Better Therapeutic Options.Cancers (Basel). 2023 Apr 27;15(9):2515. doi: 10.3390/cancers15092515. Cancers (Basel). 2023. PMID: 37173981 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

