Adiponectin Inhibits NLRP3 Inflammasome Activation in Nonalcoholic Steatohepatitis via AMPK-JNK/ErK1/2-NFκB/ROS Signaling Pathways
- PMID: 33251225
- PMCID: PMC7674946
- DOI: 10.3389/fmed.2020.546445
Adiponectin Inhibits NLRP3 Inflammasome Activation in Nonalcoholic Steatohepatitis via AMPK-JNK/ErK1/2-NFκB/ROS Signaling Pathways
Abstract
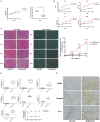
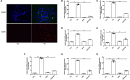
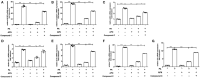
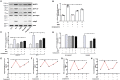
Adiponectin, an adipose-derived adipokine, possesses a hepatoprotective role in various liver disorders. It has been reported that hypoadiponectinemia can affect with the progression of non-alcoholic fatty liver diseases (NAFLD). Inflammasome activation has been recognized to play a major role during the progression of NAFLD. This research aimed to explore the effect of adiponectin on palmitate (PA)-mediated NLRP3 inflammasome activation and its potential molecular mechanisms. Male adiponectin-knockout (adiponectin-KO) mice and C57BL/6 (wild-type) mice were fed a high-fat-diet (HFD) for 12 weeks as an in vivo model of non-alcoholic steatohepatitis (NASH). Serum biochemical markers, liver histology and inflammasome-related gene and protein expression were determined. In addition, the hepatocytes isolated from wide type mice were exposed to PA in the absence or presence of adiponectin and/or AMPK inhibitor. The activation of NLRP3 inflammasome was assessed by mRNA and protein expression. Furthermore, ROS production and related signaling pathways were also evaluated. In the in vivo experiments, excessive hepatic steatosis with increased NLRP3 inflammasome and its complex expression were found in adiponectin-KO mice compared to wild-type mice. Moreover, the expression levels of NLRP3 inflammasome pathway molecules (NFκB and ROS) were upregulated, while the phosphorylation levels of AMPK, JNK, and Erk1/2 were downregulated in adiponectin-KO mice compared with wild-type mice. In the in vitro study, PA increased lipid droplet deposition, NF-kB signaling and ROS production. Additionally, PA significantly promoted NLRP3 inflammasome activation and complex gene and protein expression in hepatocytes. Adiponectin could abolish PA-mediated inflammasome activation and decrease ROS production, which was reversed by AMPK inhibitor (compound C). Furthermore, the results showed that the inhibitory effect of adiponectin on PA-mediated inflammasome activation was regulated by AMPK-JNK/ErK1/2-NFκB/ROS signaling pathway. Adiponectin inhibited PA-mediated NLRP3 inflammasome activation in hepatocytes. Adiponectin analogs or AMPK agonists could serve as a potential novel agent for preventing or delaying the progression of NASH and NAFLD.
Keywords: AMPK; NAFLD; NLRP3 inflamamasome; adiponectin; hepatocytes.
Copyright © 2020 Dong, Zhuang, Ye, Ning, Wu, Lu and Wan.
Figures

Similar articles
-
Inhibition of NLRP3 inflammasome by thioredoxin-interacting protein in mouse Kupffer cells as a regulatory mechanism for non-alcoholic fatty liver disease development.Onco_target. 2017 Jun 6;8(23):37657-37672. doi: 10.18632/onco_target.17489. Onco_target. 2017. PMID: 28499273 Free PMC article.
-
Effects of XIAP on high fat diet-induced hepatic steatosis: a mechanism involving NLRP3 inflammasome and oxidative stress.Aging (Albany NY). 2019 Dec 16;11(24):12177-12201. doi: 10.18632/aging.102559. Epub 2019 Dec 16. Aging (Albany NY). 2019. PMID: 31841118 Free PMC article.
-
NLRP3 inflammasome activation is required for fibrosis development in NAFLD.J Mol Med (Berl). 2014 Oct;92(10):1069-82. doi: 10.1007/s00109-014-1170-1. Epub 2014 May 28. J Mol Med (Berl). 2014. PMID: 24861026 Free PMC article.
-
The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic _targets and Treatment.Front Pharmacol. 2022 Mar 8;13:780496. doi: 10.3389/fphar.2022.780496. eCollection 2022. Front Pharmacol. 2022. PMID: 35350750 Free PMC article. Review.
-
Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH.Can J Gastroenterol Hepatol. 2016;2016:6489012. doi: 10.1155/2016/6489012. Epub 2016 May 4. Can J Gastroenterol Hepatol. 2016. PMID: 27446858 Free PMC article. Review.
Cited by
-
Adiponectin inhibits ROS/NLRP3 inflammatory pathway through FOXO3A to ameliorate oral submucosal fibrosis.Odontology. 2024 Jul;112(3):811-825. doi: 10.1007/s10266-023-00891-0. Epub 2024 Jan 13. Odontology. 2024. PMID: 38217790
-
What Does Sarcopenia Have to Do with Nonalcoholic Fatty Liver Disease?Life (Basel). 2023 Dec 25;14(1):37. doi: 10.3390/life14010037. Life (Basel). 2023. PMID: 38255652 Free PMC article. Review.
-
GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects.Front Endocrinol (Lausanne). 2021 Aug 23;12:721135. doi: 10.3389/fendo.2021.721135. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34497589 Free PMC article. Review.
-
AMPK signaling inhibits the differentiation of myofibroblasts: impact on age-related tissue fibrosis and degeneration.Biogerontology. 2024 Feb;25(1):83-106. doi: 10.1007/s10522-023-10072-9. Epub 2023 Nov 2. Biogerontology. 2024. PMID: 37917219 Free PMC article. Review.
-
The mysterious association between adiponectin and endometriosis.Front Pharmacol. 2024 May 15;15:1396616. doi: 10.3389/fphar.2024.1396616. eCollection 2024. Front Pharmacol. 2024. PMID: 38813109 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

