Histone tails as signaling antennas of chromatin
- PMID: 33279866
- PMCID: PMC8096652
- DOI: 10.1016/j.sbi.2020.10.018
Histone tails as signaling antennas of chromatin
Abstract
Histone tails, representing the N-terminal or C-terminal regions flanking the histone core, play essential roles in chromatin signaling networks. Intrinsic disorder of histone tails and their propensity for post-translational modifications allow them to serve as hubs in coordination of epigenetic processes within the nucleosomal context. Deposition of histone variants with distinct histone tail properties further enriches histone tails' repertoire in epigenetic signaling. Given the advances in experimental techniques and in silico modelling, we review the most recent data on histone tails' effects on nucleosome stability and dynamics, their function in regulating chromatin accessibility and folding. Finally, we discuss different molecular mechanisms to understand how histone tails are involved in nucleosome recognition by binding partners and formation of higher-order chromatin structures.
Published by Elsevier Ltd.
Figures
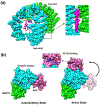
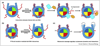
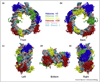
Similar articles
-
Secondary structures of the core histone N-terminal tails: their role in regulating chromatin structure.Subcell Biochem. 2013;61:37-55. doi: 10.1007/978-94-007-4525-4_2. Subcell Biochem. 2013. PMID: 23150245 Review.
-
Histone H3 and H4 tails play an important role in nucleosome phase separation.Biophys Chem. 2022 Apr;283:106767. doi: 10.1016/j.bpc.2022.106767. Epub 2022 Feb 2. Biophys Chem. 2022. PMID: 35158124 Free PMC article.
-
The effect of epigenetic modifications on the secondary structures and possible binding positions of the N-terminal tail of histone H3 in the nucleosome: a computational study.J Mol Model. 2017 Apr;23(4):137. doi: 10.1007/s00894-017-3308-x. Epub 2017 Mar 28. J Mol Model. 2017. PMID: 28353152 Free PMC article.
-
Mind the gap: Epigenetic regulation of chromatin accessibility in plants.Plant Physiol. 2024 Mar 29;194(4):1998-2016. doi: 10.1093/plphys/kiae024. Plant Physiol. 2024. PMID: 38236303 Free PMC article. Review.
-
Histone tails cooperate to control the breathing of genomic nucleosomes.PLoS Comput Biol. 2021 Jun 3;17(6):e1009013. doi: 10.1371/journal.pcbi.1009013. eCollection 2021 Jun. PLoS Comput Biol. 2021. PMID: 34081696 Free PMC article.
Cited by
-
Histone variant H2A.Z modulates nucleosome dynamics to promote DNA accessibility.Nat Commun. 2023 Feb 11;14(1):769. doi: 10.1038/s41467-023-36465-5. Nat Commun. 2023. PMID: 36765119 Free PMC article.
-
Linking metabolism and histone acetylation dynamics by integrated metabolic flux analysis of Acetyl-CoA and histone acetylation sites.Mol Metab. 2024 Dec;90:102032. doi: 10.1016/j.molmet.2024.102032. Epub 2024 Sep 19. Mol Metab. 2024. PMID: 39305948 Free PMC article.
-
What Are the Potential Roles of Nuclear Perlecan and Other Heparan Sulphate Proteoglycans in the Normal and Malignant Phenotype.Int J Mol Sci. 2021 Apr 23;22(9):4415. doi: 10.3390/ijms22094415. Int J Mol Sci. 2021. PMID: 33922532 Free PMC article. Review.
-
Priming seeds for the future: Plant immune memory and application in crop protection.Front Plant Sci. 2022 Jul 29;13:961840. doi: 10.3389/fpls.2022.961840. eCollection 2022. Front Plant Sci. 2022. PMID: 35968080 Free PMC article. Review.
-
Conformational Dynamics of the Nucleosomal Histone H2B Tails Revealed by Molecular Dynamics Simulations.J Chem Inf Model. 2024 Jun 24;64(12):4709-4726. doi: 10.1021/acs.jcim.4c00059. Epub 2024 Jun 12. J Chem Inf Model. 2024. PMID: 38865599 Free PMC article.
References
-
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ: Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389:251–260. - PubMed
-
-
Pilotto S, Speranzini V, Tortorici M, Durand D, Fish A, Valente S, Forneris F, Mai A, Sixma TK, Vachette P, et al.: Interplay among nucleosomal DNA, histone tails, and corepressor CoREST underlies LSD1-mediated H3 demethylation. Proc Natl Acad Sci U S A 2015, 112:2752–2757.
*Authors propose a tail displacement model for LSD1-mediated H3 demethylation where the interaction between the SANT2 domain and nucleosomal DNA displaces the H3 tails from DNA and make them avaibable for capture by the LSD1 active site.
-
-
-
Morrison EA, Bowerman S, Sylvers KL, Wereszczynski J, Musselman CA: The conformation of the histone H3 tail inhibits association of the BPTF PHD finger with the nucleosome. Elife 2018, 7.
**A study demonstrates that H3 tails extensively interact with nucleosome DNA which inhibits the binding activity of PHD finger to histone tail. It further shows that histone modifications and mutations weaken the tail-DNA interactions and increase the accessibiliy of histone tail.
-
-
- Stutzer A, Liokatis S, Kiesel A, Schwarzer D, Sprangers R, Soding J, Selenko P, Fischle W: Modulations of DNA Contacts by Linker Histones and Post-translational Modifications Determine the Mobility and Modifiability of Nucleosomal H3 Tails. Mol Cell 2016, 61:247–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

