Distinct Functional Traits of Lactobacilli from Women with Asymptomatic Bacterial Vaginosis and Normal Microbiota
- PMID: 33316918
- PMCID: PMC7763271
- DOI: 10.3390/microorganisms8121949
Distinct Functional Traits of Lactobacilli from Women with Asymptomatic Bacterial Vaginosis and Normal Microbiota
Abstract
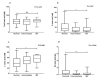
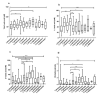
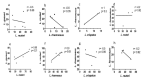
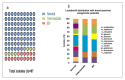
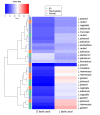
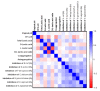
Asymptomatic bacterial vaginosis (BV) in reproductive-age women has serious obstetric and gynecological consequences. Despite its high incidence, the behavior of vaginal lactobacilli in asymptomatic BV is unknown. We analyzed the functional properties of previously isolated vaginal lactobacilli from asymptomatic women with normal, intermediate, and BV microbiota. Lactic acid and antimicrobial activity against seven urogenital pathogens were evaluated from lactobacilli cell-free culture supernatants (CFCs) (n = 207) after 48 h incubation in MRS. Lactobacilli isolates were used to evaluate H2O2, autoaggregation and coaggregation with C. albicans. Lactobacilli from normal microbiota produced more d-lactate than lactobacilli from intermediate and asymptomatic BV (p = 0.007). L. plantarum, L. fermentum and L. reuteri produced greater d-lactate whereas L. rhamnosus, L. crispatus, L. johnsonii were greater producers of l-lactate. Interspecies positive correlation was observed in the lactic acid contents of CFCs. Distribution of H2O2-producing lactobacilli did not vary significantly among the groups. When lactic acid isomers were considered, species from intermediate and BV microbiota clustered together with each other and distinctly from species of normal microbiota. Broad-spectrum antagonism (≥90% inhibition) against E. coli, C. albicans, S. aureus, P. aeruginosa, G. vaginalis, N. gonorrhoeae, S. agalactiae were displayed by 46.86% (97) of isolates. Our study highlights the differential functional properties of vaginal lactobacilli from women with normal microbiota and asymptomatic BV.
Keywords: Lactobacillus; asymptomatic BV; bacterial vaginosis; lactic acid; probiotic properties; vaginal microbiota.
Conflict of interest statement
The authors declare that there are no conflict of interest in the present study.
Figures



Similar articles
-
Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: a pilot study.J Clin Gastroenterol. 2014 Nov-Dec;48 Suppl 1:S106-12. doi: 10.1097/MCG.0000000000000226. J Clin Gastroenterol. 2014. PMID: 25291116 Clinical Trial.
-
Vaginal microbiota of asymptomatic bacterial vaginosis and vulvovaginal candidiasis: Are they different from normal microbiota?Microb Pathog. 2019 Sep;134:103599. doi: 10.1016/j.micpath.2019.103599. Epub 2019 Jun 15. Microb Pathog. 2019. PMID: 31212037
-
Identification and characterisation of vaginal lactobacilli from South African women.BMC Infect Dis. 2013 Jan 26;13:43. doi: 10.1186/1471-2334-13-43. BMC Infect Dis. 2013. PMID: 23351177 Free PMC article.
-
Probiotics for the treatment of women with bacterial vaginosis.Clin Microbiol Infect. 2007 Jul;13(7):657-64. doi: 10.1111/j.1469-0691.2007.01688.x. Clin Microbiol Infect. 2007. PMID: 17633390 Review.
-
Vaginal microbiome.Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English.
Cited by
-
Lactobacilli metabolites restore E-cadherin and suppress MMP9 in cervical cancer cells.Curr Res Toxicol. 2022 Sep 21;3:100088. doi: 10.1016/j.crtox.2022.100088. eCollection 2022. Curr Res Toxicol. 2022. PMID: 36176311 Free PMC article.
-
The impact of pelvic floor electrical stimulation on vaginal microbiota and immunity.Front Cell Infect Microbiol. 2022 Sep 27;12:1006576. doi: 10.3389/fcimb.2022.1006576. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36237426 Free PMC article. Clinical Trial.
-
In Vitro Screen of Lactobacilli Strains for Gastrointestinal and Vaginal Benefits.Microorganisms. 2023 Jan 28;11(2):329. doi: 10.3390/microorganisms11020329. Microorganisms. 2023. PMID: 36838294 Free PMC article.
-
[Preliminary Study on the Identification of Aerobic Vaginitis by Artificial Intelligence Analysis System].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 Mar 20;55(2):461-468. doi: 10.12182/20240360504. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38645857 Free PMC article. Chinese.
-
Intravaginal lactic acid gel versus oral metronidazole for treating women with recurrent bacterial vaginosis: the VITA randomised controlled trial.BMC Womens Health. 2023 May 9;23(1):241. doi: 10.1186/s12905-023-02303-5. BMC Womens Health. 2023. PMID: 37161454 Free PMC article. Clinical Trial.
References
-
- Koumans E.H., Sternberg M., Bruce C., McQuillan G., Kendrick J., Sutton M., Markowitz L.E. The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations with Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 2007;34:864–869. doi: 10.1097/OLQ.0b013e318074e565. - DOI - PubMed
-
- Joyisa N., Moodley D., Nkosi T., Talakgale R., Sebitloane M., Naidoo M., Karim Q.A. Asymptomatic Bacterial Vaginosis in Pregnancy and Missed Opportunities for Treatment: A Cross-Sectional Observational Study. Infect. Dis. Obstet. Gynecol. 2019;2019:7808179. doi: 10.1155/2019/7808179. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

