Voltage-Gated Potassium Channels as Regulators of Cell Death
- PMID: 33381507
- PMCID: PMC7767978
- DOI: 10.3389/fcell.2020.611853
Voltage-Gated Potassium Channels as Regulators of Cell Death
Abstract
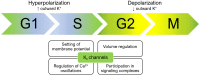
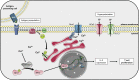
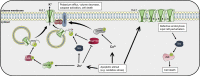
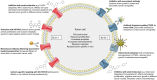
Ion channels allow the flux of specific ions across biological membranes, thereby determining ion homeostasis within the cells. Voltage-gated potassium-selective ion channels crucially contribute to the setting of the plasma membrane potential, to volume regulation and to the physiologically relevant modulation of intracellular potassium concentration. In turn, these factors affect cell cycle progression, proliferation and apoptosis. The present review summarizes our current knowledge about the involvement of various voltage-gated channels of the Kv family in the above processes and discusses the possibility of their pharmacological _targeting in the context of cancer with special emphasis on Kv1.1, Kv1.3, Kv1.5, Kv2.1, Kv10.1, and Kv11.1.
Keywords: Kv-channels; cancer; cell death; cell proliferation; mitochondria.
Copyright © 2020 Bachmann, Li, Edwards, Ahmad, Patel, Szabo and Gulbins.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes.J Clin Invest. 1998 Jun 1;101(11):2319-30. doi: 10.1172/JCI333. J Clin Invest. 1998. PMID: 9616203 Free PMC article.
-
Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells.Circ Res. 2004 Aug 6;95(3):308-18. doi: 10.1161/01.RES.0000137173.42723.fb. Epub 2004 Jun 24. Circ Res. 2004. PMID: 15217912
-
Correlation between potassium channel expression and sensitivity to drug-induced cell death in tumor cell lines.Curr Pharm Des. 2014;20(2):189-200. doi: 10.2174/13816128113199990032. Curr Pharm Des. 2014. PMID: 23701546
-
Contribution of voltage-gated potassium channels to the regulation of apoptosis.FEBS Lett. 2010 May 17;584(10):2049-56. doi: 10.1016/j.febslet.2010.01.038. Epub 2010 Jan 25. FEBS Lett. 2010. PMID: 20102714 Review.
-
Voltage-Gated Potassium Channels Kv1.3--Potentially New Molecular _target in Cancer Diagnostics and Therapy.Adv Clin Exp Med. 2015 May-Jun;24(3):517-24. doi: 10.17219/acem/22339. Adv Clin Exp Med. 2015. PMID: 26467143 Review.
Cited by
-
The hEag1 K+ Channel Inhibitor Astemizole Stimulates Ca2+ Deposition in SaOS-2 and MG-63 Osteosarcoma Cultures.Int J Mol Sci. 2022 Sep 11;23(18):10533. doi: 10.3390/ijms231810533. Int J Mol Sci. 2022. PMID: 36142445 Free PMC article.
-
Hypoxia with inflammation and reperfusion alters membrane resistance by dynamically regulating voltage-gated potassium channels in hippocampal CA1 neurons.Mol Brain. 2021 Sep 23;14(1):147. doi: 10.1186/s13041-021-00857-9. Mol Brain. 2021. PMID: 34556177 Free PMC article.
-
Down the membrane hole: Ion channels in protozoan parasites.PLoS Pathog. 2022 Dec 29;18(12):e1011004. doi: 10.1371/journal.ppat.1011004. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36580479 Free PMC article. Review.
-
Recent Advances in Computer-Aided Structure-Based Drug Design on Ion Channels.Int J Mol Sci. 2023 May 25;24(11):9226. doi: 10.3390/ijms24119226. Int J Mol Sci. 2023. PMID: 37298178 Free PMC article. Review.
-
Enhancing Human Cutaneous Wound Healing through _targeted Suppression of Large Conductance Ca2+-Activated K+ Channels.Int J Mol Sci. 2024 Jan 9;25(2):803. doi: 10.3390/ijms25020803. Int J Mol Sci. 2024. PMID: 38255877 Free PMC article.
References
-
- Abdul M., Santo A., Hoosein N. (2003). Activity of potassium channel-blockers in breast cancer. Anticancer Res. 23 3347–3351. - PubMed
-
- Aissaoui D., Mlayah-Bellalouna S., Jebali J., Abdelkafi-Koubaa Z., Souid S., Moslah W., et al. (2018). Functional role of Kv1.1 and Kv1.3 channels in the neoplastic progression steps of three cancer cell lines, elucidated by scorpion peptides. Int. J. Biol. Macromol. 111 1146–1155. 10.1016/j.ijbiomac.2018.01.144 - DOI - PubMed
-
- Al-Owais M. M., Scragg J. L., Dallas M. L., Boycott H. E., Warburton P., Chakrabarty A., et al. (2012). Carbon monoxide mediates the anti-apoptotic effects of heme oxygenase-1 in medulloblastoma DAOY cells via K+ channel inhibition. J. Biol. Chem. 287 24754–24764. 10.1074/jbc.M112.357012 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

