Epidemiological Challenges in Rare Bleeding Disorders: FVIII Inhibitor Incidence in Haemophilia A Patients-A Known Issue of Unknown Origin
- PMID: 33396748
- PMCID: PMC7795862
- DOI: 10.3390/ijerph18010225
Epidemiological Challenges in Rare Bleeding Disorders: FVIII Inhibitor Incidence in Haemophilia A Patients-A Known Issue of Unknown Origin
Abstract
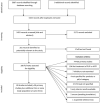
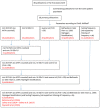
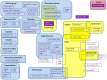
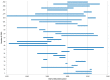
There is a broad range of factor products approved in Germany for haemophilia A treatment. Since the introduction of recombinant coagulation factor VIII (FVIII) products in the 1990s, there has been substantial debate whether there is a difference in inhibitor incidence between single FVIII products or product classes. Neither haemophilia registries nor clinical studies, including a randomised controlled clinical trial, provided a consistent and definite answer. The reasons were mainly related to methodological challenges in conducting controlled studies in a rare disease. In this analysis, the most relevant epidemiological challenges and main problems were examined, including study bias, potential overlap of individual studies and advanced development of therapy and methods in the course of time. Meta-analyses on two levels showed that therapies using recombinant products resulted in different event rates when compared to plasma-derived products. These results are accompanied by substantial study heterogeneity evidenced by Cochran's Q tests. Only three studies have been identified that meet the standards of current clinical guidance. To finally resolve this ongoing and disputable safety issue of replacement therapy, collaboration among registry owners, academia and regulators must be fostered.
Keywords: epidemiology; haemophilia A; inhibitor development; rare diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Prospective surveillance study of haemophilia A patients switching from moroctocog alfa or other factor VIII products to moroctocog alfa albumin-free cell culture (AF-CC) in usual care settings.Thromb Haemost. 2015 Oct;114(4):676-84. doi: 10.1160/TH14-09-0760. Epub 2015 Aug 13. Thromb Haemost. 2015. PMID: 26293201 Clinical Trial.
-
Prospective observational cohort studies for studying rare diseases: the European PedNet Haemophilia Registry.Haemophilia. 2014 Jul;20(4):e280-6. doi: 10.1111/hae.12448. Epub 2014 May 2. Haemophilia. 2014. PMID: 24784937
-
Treating haemophilia A with recombinant blood factors: a comparison.Expert Opin Pharmacother. 2004 May;5(5):1061-70. doi: 10.1517/14656566.5.5.1061. Expert Opin Pharmacother. 2004. PMID: 15155108 Review.
-
Recombinant factor VIII products and inhibitor development in previously untreated patients with severe haemophilia A: Combined analysis of three studies.Haemophilia. 2019 May;25(3):398-407. doi: 10.1111/hae.13747. Epub 2019 May 7. Haemophilia. 2019. PMID: 31066174 Review.
-
[Inhibitor development after changing FVIII/IX products in patients with haemophilia].Hamostaseologie. 2012;32 Suppl 1:S39-42. Hamostaseologie. 2012. PMID: 22961330 German.
Cited by
-
Predictors of the outcome of immune tolerance induction in patients with haemophilia A and inhibitors: The Brazilian Immune Tolerance (BrazIT) Study protocol.PLoS One. 2021 Aug 26;16(8):e0256265. doi: 10.1371/journal.pone.0256265. eCollection 2021. PLoS One. 2021. PMID: 34437573 Free PMC article.
-
Progress in the Development of Anti-tissue Factor Pathway Inhibitors for Haemophilia Management.Front Med (Lausanne). 2021 May 5;8:670526. doi: 10.3389/fmed.2021.670526. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34026796 Free PMC article. Review.
References
-
- Gouw S.C., Berg H.M.V.D., Fischer K., Auerswald G., Carcao M., Chalmers E., Chambost H., Kurnik K., Liesner R.I., Petrini P., et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: The RODIN study. Blood. 2013;121:4046–4055. doi: 10.1182/blood-2012-09-457036. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

