The Source of Glycolytic Intermediates in Mammalian Tissues
- PMID: 33472024
- PMCID: PMC8088818
- DOI: 10.1016/j.cmet.2020.12.020
The Source of Glycolytic Intermediates in Mammalian Tissues
Abstract
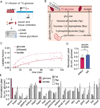
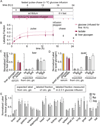
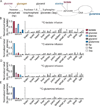
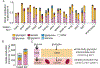
Glycolysis plays a central role in organismal metabolism, but its quantitative inputs across mammalian tissues remain unclear. Here we use 13C-tracing in mice to quantify glycolytic intermediate sources: circulating glucose, intra-tissue glycogen, and circulating gluconeogenic precursors. Circulating glucose is the main source of circulating lactate, the primary end product of tissue glycolysis. Yet circulating glucose highly labels glycolytic intermediates in only a few tissues: blood, spleen, diaphragm, and soleus muscle. Most glycolytic intermediates in the bulk of body tissue, including liver and quadriceps muscle, come instead from glycogen. Gluconeogenesis contributes less but also broadly to glycolytic intermediates, and its flux persists with physiologic feeding (but not hyperinsulinemic clamp). Instead of suppressing gluconeogenesis, feeding activates oxidation of circulating glucose and lactate to maintain glucose homeostasis. Thus, the bulk of the body slowly breaks down internally stored glycogen while select tissues rapidly catabolize circulating glucose to lactate for oxidation throughout the body.
Keywords: compartmentalized metabolism; glucose homeostasis; glycogen; glycolysis; glycolytic intermediates; glycolytic specialist; isotope tracing; metabolic heterogeneity; red muscle.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests J.D.R. is an advisor and stockholder in Colorado Research Partners, a paid consultant of Pfizer, a founder and stockholder in Toran Therapeutics, and inventor of patents held by Princeton University.
Figures



Similar articles
-
Short-term modulation of glycogen metabolism, glycolysis and gluconeogenesis by physiological oxygen concentrations in hepatocyte cultures.Eur J Biochem. 1983 Oct 3;135(3):405-12. doi: 10.1111/j.1432-1033.1983.tb07667.x. Eur J Biochem. 1983. PMID: 6413204
-
Formation of gluconeogenic precursors in rat skeletal muscle during fasted-refed transition.Am J Physiol. 1990 Oct;259(4 Pt 1):E513-6. doi: 10.1152/ajpendo.1990.259.4.E513. Am J Physiol. 1990. PMID: 2221052
-
Differential effects of safflower oil versus fish oil feeding on insulin-stimulated glycogen synthesis, glycolysis, and pyruvate dehydrogenase flux in skeletal muscle: a 13C nuclear magnetic resonance study.Diabetes. 1999 Jan;48(1):134-40. doi: 10.2337/diabetes.48.1.134. Diabetes. 1999. PMID: 9892234
-
The lactate shuttle during exercise and recovery.Med Sci Sports Exerc. 1986 Jun;18(3):360-8. doi: 10.1249/00005768-198606000-00019. Med Sci Sports Exerc. 1986. PMID: 3523107 Review.
-
Control of glycaemia.Baillieres Clin Endocrinol Metab. 1993 Jul;7(3):551-86. doi: 10.1016/s0950-351x(05)80207-1. Baillieres Clin Endocrinol Metab. 1993. PMID: 8379904 Review.
Cited by
-
KCNJ16-depleted kidney organoids recapitulate tubulopathy and lipid recovery upon statins treatment.Stem Cell Res Ther. 2024 Aug 26;15(1):268. doi: 10.1186/s13287-024-03881-3. Stem Cell Res Ther. 2024. PMID: 39183338 Free PMC article.
-
Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth.Med. 2022 Feb 11;3(2):119-136. doi: 10.1016/j.medj.2021.12.008. Med. 2022. PMID: 35425930 Free PMC article.
-
The dentate gyrus differentially metabolizes glucose and alternative fuels during rest and stimulation.J Neurochem. 2024 May;168(5):533-554. doi: 10.1111/jnc.16004. Epub 2023 Nov 6. J Neurochem. 2024. PMID: 37929637
-
Intracellular energy controls dynamics of stress-induced ribonucleoprotein granules.Nat Commun. 2022 Sep 23;13(1):5584. doi: 10.1038/s41467-022-33079-1. Nat Commun. 2022. PMID: 36151083 Free PMC article.
-
Sepsis, pyruvate, and mitochondria energy supply chain shortage.J Leukoc Biol. 2022 Dec;112(6):1509-1514. doi: 10.1002/JLB.3MR0322-692RR. Epub 2022 Jul 22. J Leukoc Biol. 2022. PMID: 35866365 Free PMC article. Review.
References
-
- Augusto V, Padovani CR, and Rocha Campos GE (2004). Skeletal muscle fiber types in C57Bl6J mice. pp. 89–94.
-
- Ayala JE, Bracy DP, McGuinness OP, and Wasserman DH (2006). Considerations in the Design of Hyperinsulinemic-Euglycemic Clamps in the Conscious Mouse. Diabetes 55, 390. - PubMed
-
- Baron AD, Brechtel G, Wallace P, and Edelman SV (1988). Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. American Journal of Physiology-Endocrinology and Metabolism 255, E769–E774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

