Immune checkpoint: The novel _target for antitumor therapy
- PMID: 33569511
- PMCID: PMC7859424
- DOI: 10.1016/j.gendis.2019.12.004
Immune checkpoint: The novel _target for antitumor therapy
Abstract
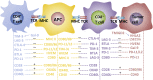
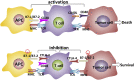
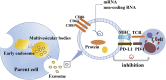
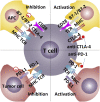
Inhibitory checkpoint molecules include programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), cytotoxic T lymphocyte antigen-4 (CTLA-4), human endogenous retrovirus-H Long terminal repeat-associating 2 (HHLA2), B7 homolog 4 protein (B7-H4), T cell membrane protein-3 (TIM-3) and Lymphocyte-activation gene 3 (LAG-3), which are up-regulated during tumorigenesis. These pathways are essential to down-regulate the immune system by blocking the activation of T cells. In recent years, immune checkpoint blockers (ICBs) against PD-1, PD-L1, CTLA-4 or TIM-3 has made remarkable progress in the clinical application, revolutionizing the treatment of malignant tumors and improving patients' overall survival. However, the efficacy of ICBs in some patients does not seem to be good enough, and more immune-related adverse events (irAEs) will inevitably occur. Therefore, biomarkers research provides practical guidance for clinicians to identify patients who are most likely to benefit from or exhibit resistance to particular types of immune checkpoint therapy. There are two points in general. On the one hand, given the spatial and temporal differential expression of immune checkpoint molecules during immunosuppression process, it is essential to understand their mechanisms to design the most effective individualized therapy. On the other hand, due to the lack of potent immune checkpoints, it is necessary to combine them with novel biomarkers (such as exosomes and ctDNA) and other anticancer modalities (such as chemotherapy and radiotherapy).
Keywords: Circulating tumor DNA (ctDNA); Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4); Exosome; Immune checkpoint; Lymphocyte-activation gene 3 (LAG-3); Programmed cell death protein ligand 1 (PD-L1); Programmed death-1 receptor (PD-1); T cell immunoglobulin domain and mucin domain 3 (TIM-3).
© 2019 Chongqing Medical University. Production and hosting by Elsevier B.V.
Figures
Similar articles
-
Manipulation of the Immune System for Cancer Defeat: A Focus on the T Cell Inhibitory Checkpoint Molecules.Curr Med Chem. 2020;27(15):2402-2448. doi: 10.2174/0929867325666181106114421. Curr Med Chem. 2020. PMID: 30398102 Review.
-
Role of Next Generation Immune Checkpoint Inhibitor (ICI) Therapy in Philadelphia Negative Classic Myeloproliferative Neoplasm (MPN): Review of the Literature.Int J Mol Sci. 2023 Aug 7;24(15):12502. doi: 10.3390/ijms241512502. Int J Mol Sci. 2023. PMID: 37569880 Free PMC article. Review.
-
Clinical Insights Into Novel Immune Checkpoint Inhibitors.Front Pharmacol. 2021 May 6;12:681320. doi: 10.3389/fphar.2021.681320. eCollection 2021. Front Pharmacol. 2021. PMID: 34025438 Free PMC article. Review.
-
Immune Co-inhibitory Receptors PD-1, CTLA-4, TIM-3, LAG-3, and TIGIT in Medullary Thyroid Cancers: A Large Cohort Study.J Clin Endocrinol Metab. 2021 Jan 1;106(1):120-132. doi: 10.1210/clinem/dgaa701. J Clin Endocrinol Metab. 2021. PMID: 33000173
-
Novel immune checkpoint _targets: moving beyond PD-1 and CTLA-4.Mol Cancer. 2019 Nov 6;18(1):155. doi: 10.1186/s12943-019-1091-2. Mol Cancer. 2019. PMID: 31690319 Free PMC article. Review.
Cited by
-
Checkpoint inhibitors as immunotherapy for fungal infections: Promises, challenges, and unanswered questions.Front Immunol. 2022 Oct 25;13:1018202. doi: 10.3389/fimmu.2022.1018202. eCollection 2022. Front Immunol. 2022. PMID: 36389687 Free PMC article. Review.
-
Cell-free chromatin particles released from dying cancer cells activate immune checkpoints in human lymphocytes: implications for cancer therapy.Front Immunol. 2024 Jan 11;14:1331491. doi: 10.3389/fimmu.2023.1331491. eCollection 2023. Front Immunol. 2024. PMID: 38274821 Free PMC article.
-
Stable triangle: nanomedicine-based synergistic application of phototherapy and immunotherapy for tumor treatment.J Nanobiotechnology. 2024 Oct 18;22(1):635. doi: 10.1186/s12951-024-02925-3. J Nanobiotechnology. 2024. PMID: 39420366 Free PMC article. Review.
-
LncRNA MEG3 promotes chemosensitivity of osteosarcoma by regulating antitumor immunity via miR-21-5p/p53 pathway and autophagy.Genes Dis. 2021 Nov 24;10(2):531-541. doi: 10.1016/j.gendis.2021.11.004. eCollection 2023 Mar. Genes Dis. 2021. PMID: 37223512 Free PMC article.
-
Emerging druggable _targets for immune checkpoint modulation in cancer immunotherapy: the iceberg lies beneath the surface.Apoptosis. 2024 Dec;29(11-12):1879-1913. doi: 10.1007/s10495-024-02022-8. Epub 2024 Oct 1. Apoptosis. 2024. PMID: 39354213 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

