Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials
- PMID: 33617777
- PMCID: PMC7894131
- DOI: 10.1016/S0140-6736(21)00432-3
Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials
Erratum in
-
Department of Error.Lancet. 2021 Mar 6;397(10277):880. doi: 10.1016/S0140-6736(21)00515-8. Lancet. 2021. PMID: 33676625 Free PMC article. No abstract available.
Abstract
Background: The ChAdOx1 nCoV-19 (AZD1222) vaccine has been approved for emergency use by the UK regulatory authority, Medicines and Healthcare products Regulatory Agency, with a regimen of two standard doses given with an interval of 4-12 weeks. The planned roll-out in the UK will involve vaccinating people in high-risk categories with their first dose immediately, and delivering the second dose 12 weeks later. Here, we provide both a further prespecified pooled analysis of trials of ChAdOx1 nCoV-19 and exploratory analyses of the impact on immunogenicity and efficacy of extending the interval between priming and booster doses. In addition, we show the immunogenicity and protection afforded by the first dose, before a booster dose has been offered.
Methods: We present data from three single-blind randomised controlled trials-one phase 1/2 study in the UK (COV001), one phase 2/3 study in the UK (COV002), and a phase 3 study in Brazil (COV003)-and one double-blind phase 1/2 study in South Africa (COV005). As previously described, individuals 18 years and older were randomly assigned 1:1 to receive two standard doses of ChAdOx1 nCoV-19 (5 × 1010 viral particles) or a control vaccine or saline placebo. In the UK trial, a subset of participants received a lower dose (2·2 × 1010 viral particles) of the ChAdOx1 nCoV-19 for the first dose. The primary outcome was virologically confirmed symptomatic COVID-19 disease, defined as a nucleic acid amplification test (NAAT)-positive swab combined with at least one qualifying symptom (fever ≥37·8°C, cough, shortness of breath, or anosmia or ageusia) more than 14 days after the second dose. Secondary efficacy analyses included cases occuring at least 22 days after the first dose. Antibody responses measured by immunoassay and by pseudovirus neutralisation were exploratory outcomes. All cases of COVID-19 with a NAAT-positive swab were adjudicated for inclusion in the analysis by a masked independent endpoint review committee. The primary analysis included all participants who were SARS-CoV-2 N protein seronegative at baseline, had had at least 14 days of follow-up after the second dose, and had no evidence of previous SARS-CoV-2 infection from NAAT swabs. Safety was assessed in all participants who received at least one dose. The four trials are registered at ISRCTN89951424 (COV003) and ClinicalTrials.gov, NCT04324606 (COV001), NCT04400838 (COV002), and NCT04444674 (COV005).
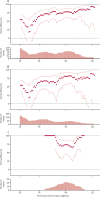
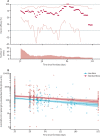
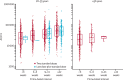
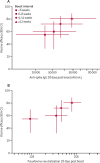
Findings: Between April 23 and Dec 6, 2020, 24 422 participants were recruited and vaccinated across the four studies, of whom 17 178 were included in the primary analysis (8597 receiving ChAdOx1 nCoV-19 and 8581 receiving control vaccine). The data cutoff for these analyses was Dec 7, 2020. 332 NAAT-positive infections met the primary endpoint of symptomatic infection more than 14 days after the second dose. Overall vaccine efficacy more than 14 days after the second dose was 66·7% (95% CI 57·4-74·0), with 84 (1·0%) cases in the 8597 participants in the ChAdOx1 nCoV-19 group and 248 (2·9%) in the 8581 participants in the control group. There were no hospital admissions for COVID-19 in the ChAdOx1 nCoV-19 group after the initial 21-day exclusion period, and 15 in the control group. 108 (0·9%) of 12 282 participants in the ChAdOx1 nCoV-19 group and 127 (1·1%) of 11 962 participants in the control group had serious adverse events. There were seven deaths considered unrelated to vaccination (two in the ChAdOx1 nCov-19 group and five in the control group), including one COVID-19-related death in one participant in the control group. Exploratory analyses showed that vaccine efficacy after a single standard dose of vaccine from day 22 to day 90 after vaccination was 76·0% (59·3-85·9). Our modelling analysis indicated that protection did not wane during this initial 3-month period. Similarly, antibody levels were maintained during this period with minimal waning by day 90 (geometric mean ratio [GMR] 0·66 [95% CI 0·59-0·74]). In the participants who received two standard doses, after the second dose, efficacy was higher in those with a longer prime-boost interval (vaccine efficacy 81·3% [95% CI 60·3-91·2] at ≥12 weeks) than in those with a short interval (vaccine efficacy 55·1% [33·0-69·9] at <6 weeks). These observations are supported by immunogenicity data that showed binding antibody responses more than two-fold higher after an interval of 12 or more weeks compared with an interval of less than 6 weeks in those who were aged 18-55 years (GMR 2·32 [2·01-2·68]).
Interpretation: The results of this primary analysis of two doses of ChAdOx1 nCoV-19 were consistent with those seen in the interim analysis of the trials and confirm that the vaccine is efficacious, with results varying by dose interval in exploratory analyses. A 3-month dose interval might have advantages over a programme with a short dose interval for roll-out of a pandemic vaccine to protect the largest number of individuals in the population as early as possible when supplies are scarce, while also improving protection after receiving a second dose.
Funding: UK Research and Innovation, National Institutes of Health Research (NIHR), The Coalition for Epidemic Preparedness Innovations, the Bill & Melinda Gates Foundation, the Lemann Foundation, Rede D'Or, the Brava and Telles Foundation, NIHR Oxford Biomedical Research Centre, Thames Valley and South Midland's NIHR Clinical Research Network, and AstraZeneca.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Single-dose Oxford-AstraZeneca COVID-19 vaccine followed by a 12-week booster.Lancet. 2021 Mar 6;397(10277):854-855. doi: 10.1016/S0140-6736(21)00528-6. Lancet. 2021. PMID: 33676614 Free PMC article. No abstract available.
-
The potential risks of delaying the second vaccine dose during the SARS-CoV-2 pandemic.QJM. 2021 May 19;114(3):163-165. doi: 10.1093/qjmed/hcab046. QJM. 2021. PMID: 33677593 Free PMC article. No abstract available.
-
ChAdOx1 nCoV-19 vaccine: asymptomatic efficacy estimates.Lancet. 2021 Jun 12;397(10291):2247-2248. doi: 10.1016/S0140-6736(21)00951-X. Lancet. 2021. PMID: 34119058 No abstract available.
-
ChAdOx1 nCoV-19 vaccine: asymptomatic efficacy estimates - Authors' reply.Lancet. 2021 Jun 12;397(10291):2248. doi: 10.1016/S0140-6736(21)00976-4. Lancet. 2021. PMID: 34119059 No abstract available.
Similar articles
-
Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial.Lancet HIV. 2021 Sep;8(9):e568-e580. doi: 10.1016/S2352-3018(21)00157-0. Epub 2021 Aug 17. Lancet HIV. 2021. PMID: 34416193 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of an AS03-adjuvanted SARS-CoV-2 recombinant protein vaccine (CoV2 preS dTM) in healthy adults: interim findings from a phase 2, randomised, dose-finding, multicentre study.Lancet Infect Dis. 2022 May;22(5):636-648. doi: 10.1016/S1473-3099(21)00764-7. Epub 2022 Jan 25. Lancet Infect Dis. 2022. PMID: 35090638 Free PMC article. Clinical Trial.
-
Safety and efficacy of PfSPZ Vaccine against malaria in healthy adults and women anticipating pregnancy in Mali: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials.Lancet Infect Dis. 2024 Dec;24(12):1366-1382. doi: 10.1016/S1473-3099(24)00360-8. Epub 2024 Aug 14. Lancet Infect Dis. 2024. PMID: 39153490 Clinical Trial.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
-
Tamoxifen for adults with hepatocellular carcinoma.Cochrane Database Syst Rev. 2024 Aug 12;8(8):CD014869. doi: 10.1002/14651858.CD014869.pub2. Cochrane Database Syst Rev. 2024. PMID: 39132750 Review.
Cited by
-
The Next Set of COVID-19 Vaccines: Leveraging New Development Platforms to Increase Access for More People Around the World.Clin Ther. 2021 Apr;43(4):702-710. doi: 10.1016/j.clinthera.2021.03.007. Epub 2021 Mar 23. Clin Ther. 2021. PMID: 33832783 Free PMC article. Review.
-
COVID-19: vaccine's progress.Microb Biotechnol. 2021 Jul;14(4):1246-1257. doi: 10.1111/1751-7915.13818. Epub 2021 May 7. Microb Biotechnol. 2021. PMID: 33960659 Free PMC article.
-
Nano-multilamellar lipid vesicles promote the induction of SARS-CoV-2 immune responses by a protein-based vaccine formulation.Nanomedicine. 2022 Sep;45:102595. doi: 10.1016/j.nano.2022.102595. Epub 2022 Aug 27. Nanomedicine. 2022. PMID: 36031045 Free PMC article.
-
Infectious viral shedding of SARS-CoV-2 Delta following vaccination: A longitudinal cohort study.PLoS Pathog. 2022 Sep 12;18(9):e1010802. doi: 10.1371/journal.ppat.1010802. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36095030 Free PMC article.
-
Concerns about SARS-CoV-2 evolution should not hold back efforts to expand vaccination.Nat Rev Immunol. 2021 May;21(5):330-335. doi: 10.1038/s41577-021-00544-9. Epub 2021 Apr 1. Nat Rev Immunol. 2021. PMID: 33795856 Free PMC article. Review.
References
-
- WHO The COVID-19 candidate vaccine landscape. Feb 16, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand...
-
- UK Department of Health and Social Care Statement from the UK Chief Medical Officers on the prioritisation of first doses of COVID-19 vaccines. Dec 30, 2020. https://www.gov.uk/government/news/statement-from-the-uk-chief-medical-o...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- MC_UU_00004/04/MRC_/Medical Research Council/United Kingdom
- MR/L006588/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_18058/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/22/MRC_/Medical Research Council/United Kingdom
- MC_PC_19058/MRC_/Medical Research Council/United Kingdom
- 211153/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_19055/MRC_/Medical Research Council/United Kingdom
- 109965/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_00008/11/MRC_/Medical Research Council/United Kingdom
- MC_PC_19026/MRC_/Medical Research Council/United Kingdom
- MR/L018942/1/MRC_/Medical Research Council/United Kingdom
- G0600520/MRC_/Medical Research Council/United Kingdom
- G1001046/MRC_/Medical Research Council/United Kingdom
- MR/N008219/1/MRC_/Medical Research Council/United Kingdom
- MR/T031891/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

