Tumor-derived ILT4 induces T cell senescence and suppresses tumor immunity
- PMID: 33653799
- PMCID: PMC7929805
- DOI: 10.1136/jitc-2020-001536
Tumor-derived ILT4 induces T cell senescence and suppresses tumor immunity
Abstract
Background: Current immunotherapies including checkpoint blockade therapy have limited success rates in certain types of cancers. Identification of alternative checkpoint molecules for the development of effective strategies for tumor immunotherapy is urgently needed. Immunoglobulin-like transcript 4 (ILT4) is an immunosuppressive molecule expressed in both myeloid innate cells and malignant tumor cells. However, the role of tumor-derived ILT4 in regulating cancer biology and tumor immunity remains unclear.
Methods: ILT4 expression in tumor cells and patient samples was determined by real-time PCR, flow cytometry, and immunohistochemistry. T cell senescence induced by tumor was evaluated using multiple markers and assays. Moreover, metabolic enzyme and signaling molecule expression and lipid droplets in tumor cells were determined using real-time PCR, western blot and oil red O staining, respectively. Loss-of-function and gain-of-function strategies were used to identify the causative role of ILT4 in tumor-induced T cell senescence. In addition, breast cancer and melanoma mouse tumor models were performed to demonstrate the role of ILT4 as a checkpoint molecule for tumor immunotherapy.
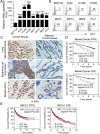
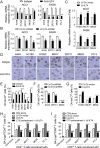
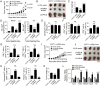
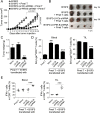
Results: We reported that ILT4 is highly expressed in human tumor cells and tissues, which is negatively associated with clinical outcomes. Furthermore, tumor-derived ILT4/PIR-B (ILT4 ortholog in mouse) is directly involved in induction of cell senescence in naïve/effector T cells mediated by tumor cells in vitro and in vivo. Mechanistically, ILT4/PIR-B increases fatty acid synthesis and lipid accumulation in tumor cells via activation of MAPK ERK1/2 signaling, resulting in promotion of tumor growth and progression, and induction of effector T cell senescence. In addition, blocking tumor-derived PIR-B can reprogram tumor metabolism, prevent senescence development in tumor-specific T cells, and enhance antitumor immunity in both breast cancer and melanoma mouse models.
Conclusions: These studies identify a novel mechanism responsible for ILT4-mediated immune suppression in the tumor microenvironment, and prove a novel concept of ILT4 as a critical checkpoint molecule for tumor immunotherapy.
Keywords: adoptive; biomarkers; immune evation; immunotherapy; lymphocytes; tumor; tumor microenvironment; tumor-Infiltrating.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation.Theranostics. 2021 Jan 19;11(7):3392-3416. doi: 10.7150/thno.52435. eCollection 2021. Theranostics. 2021. PMID: 33537094 Free PMC article.
-
ILT4 functions as a potential checkpoint molecule for tumor immunotherapy.Biochim Biophys Acta Rev Cancer. 2018 Apr;1869(2):278-285. doi: 10.1016/j.bbcan.2018.04.001. Epub 2018 Apr 10. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29649510 Review.
-
Blockades of effector T cell senescence and exhaustion synergistically enhance antitumor immunity and immunotherapy.J Immunother Cancer. 2022 Oct;10(10):e005020. doi: 10.1136/jitc-2022-005020. J Immunother Cancer. 2022. PMID: 36192086 Free PMC article.
-
Inhibitory receptor immunoglobulin-like transcript 4 was highly expressed in primary ductal and lobular breast cancer and significantly correlated with IL-10.Diagn Pathol. 2014 Apr 24;9:85. doi: 10.1186/1746-1596-9-85. Diagn Pathol. 2014. PMID: 24762057 Free PMC article.
-
Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment.Cell Mol Immunol. 2020 Jan;17(1):27-35. doi: 10.1038/s41423-019-0344-8. Epub 2019 Dec 18. Cell Mol Immunol. 2020. PMID: 31853000 Free PMC article. Review.
Cited by
-
The Zeb1-Cxcl1 axis impairs the antitumor immune response by inducing M2 macrophage polarization in breast cancer.Am J Cancer Res. 2024 Sep 15;14(9):4378-4397. doi: 10.62347/UAIS7070. eCollection 2024. Am J Cancer Res. 2024. PMID: 39417185 Free PMC article.
-
Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers.Mol Cancer. 2023 Mar 21;22(1):58. doi: 10.1186/s12943-023-01725-x. Mol Cancer. 2023. PMID: 36941614 Free PMC article. Review.
-
Elucidating the role of S100A10 in CD8+ T cell exhaustion and HCC immune escape via the cPLA2 and 5-LOX axis.Cell Death Dis. 2024 Aug 8;15(8):573. doi: 10.1038/s41419-024-06895-0. Cell Death Dis. 2024. PMID: 39117605 Free PMC article.
-
Reprogramming of Lipid Metabolism Mediates Crosstalk, Remodeling, and Intervention of Microenvironment Components in Breast Cancer.Int J Biol Sci. 2024 Mar 3;20(5):1884-1904. doi: 10.7150/ijbs.92125. eCollection 2024. Int J Biol Sci. 2024. PMID: 38481820 Free PMC article. Review.
-
Integrating Single-Cell RNA-Seq and Bulk RNA-Seq Data to Explore the Key Role of Fatty Acid Metabolism in Breast Cancer.Int J Mol Sci. 2023 Aug 25;24(17):13209. doi: 10.3390/ijms241713209. Int J Mol Sci. 2023. PMID: 37686016 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
