Role of Antimicrobial Susceptibility Testing before First-Line Treatment Containing Clarithromycin for Helicobacter pylori Eradication in the Clinical Setting
- PMID: 33669969
- PMCID: PMC7924850
- DOI: 10.3390/antibiotics10020214
Role of Antimicrobial Susceptibility Testing before First-Line Treatment Containing Clarithromycin for Helicobacter pylori Eradication in the Clinical Setting
Abstract
Background: Checking Helicobacter pylori susceptibility tests in the clinical setting before first-line treatment is considered difficult. We compared susceptibility-guided therapy (SGT) with empirical therapy (ET) as a first-line treatment containing clarithromycin and investigated the eradication rate using antimicrobial susceptibility testing (AST).
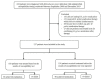
Methods: 257 patients with H. pylori infection, with AST, performed before the eradication of clarithromycin-containing regimens were enrolled and divided into two groups: the SGT and ET groups.
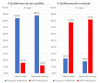
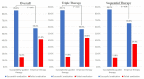
Results: Eradication rates in the SGT and ET groups were 85.4% and 58.4% (P < 0.01), respectively. In triple therapy (TT), eradication rates of the SGT and ET groups were 85.1% and 56.6% (P < 0.01), respectively. In sequential therapy (SET), eradication rates of the SGT and ET groups were 86.2% and 65.6% (P = 0.06), respectively. According to AST, TT had an eradication rate of 84.6% with strains susceptible to clarithromycin and amoxicillin and 11.1% with strains resistant to both. SET had an eradication rate of 89.5% with strains susceptible to clarithromycin, amoxicillin, and metronidazole, whereas it was 0% with strains resistant to clarithromycin and metronidazole.
Conclusions: SGT as first-line treatment improved eradication rates of TT and SET by 28.5 (P < 0.01) and 20.6 (P = 0.06) percent points, respectively, compared with ET.
Keywords: Helicobacter infection; Helicobacter pylori; clarithromycin; microbial drug resistance; microbial sensitivity tests.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains.Helicobacter. 2012 Aug;17(4):269-76. doi: 10.1111/j.1523-5378.2012.00947.x. Epub 2012 Mar 30. Helicobacter. 2012. PMID: 22759326 Clinical Trial.
-
Pretreatment antimicrobial susceptibility-guided vs. clarithromycin-based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance.Am J Gastroenterol. 2014 Oct;109(10):1595-602. doi: 10.1038/ajg.2014.222. Epub 2014 Aug 5. Am J Gastroenterol. 2014. PMID: 25091062 Clinical Trial.
-
Vonoprazan-based triple therapy is non-inferior to susceptibility-guided proton pump inhibitor-based triple therapy for Helicobacter pylori eradication.Ann Clin Microbiol Antimicrob. 2018 Jun 28;17(1):29. doi: 10.1186/s12941-018-0281-x. Ann Clin Microbiol Antimicrob. 2018. PMID: 29950163 Free PMC article.
-
Is Only Clarithromycin Susceptibility Important for the Successful Eradication of Helicobacter pylori?Antibiotics (Basel). 2020 Sep 9;9(9):589. doi: 10.3390/antibiotics9090589. Antibiotics (Basel). 2020. PMID: 32916937 Free PMC article.
-
Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review.Therap Adv Gastroenterol. 2020 Nov 12;13:1756284820968736. doi: 10.1177/1756284820968736. eCollection 2020. Therap Adv Gastroenterol. 2020. PMID: 33240392 Free PMC article. Review.
Cited by
-
Adherence to international guidelines for the management of Helicobacter pylori infection among gastroenterologists and gastroenterology fellows in Italy: A Survey of the Italian Federation of Digestive Diseases - FISMAD.Helicobacter. 2022 Feb;27(1):e12862. doi: 10.1111/hel.12862. Epub 2021 Nov 11. Helicobacter. 2022. PMID: 34766392 Free PMC article.
-
Effectiveness of 7-day triple therapy with half-dose clarithromycin for the eradication of Helicobacter pylori without the A2143G and A2142G point mutations of the 23S rRNA gene in a high clarithromycin resistance area.Front Med (Lausanne). 2023 Mar 22;10:1150396. doi: 10.3389/fmed.2023.1150396. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37035320 Free PMC article.
-
Susceptibility-guided sequential strategy versus empirical therapy for Helicobacter pylori infection: study protocol for a randomised controlled trial.Trials. 2023 Jun 19;24(1):413. doi: 10.1186/s13063-023-07457-z. Trials. 2023. PMID: 37337241 Free PMC article.
-
Comparative Efficacy of Azithromycin and Clarithromycin in the Management of Helicobacter pylori Infection.Cureus. 2024 Oct 21;16(10):e72033. doi: 10.7759/cureus.72033. eCollection 2024 Oct. Cureus. 2024. PMID: 39569309 Free PMC article.
-
Helicobacter pylori Antimicrobial Susceptibility Testing-Guided Eradication Therapy in the Southeast Region of China: A Retrospective Study.Infect Drug Resist. 2024 Nov 16;17:5079-5086. doi: 10.2147/IDR.S487503. eCollection 2024. Infect Drug Resist. 2024. PMID: 39575108 Free PMC article.
References
-
- Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. - DOI - PubMed
-
- Lim J.H., Kim N., Lim S.H., Kwon J.W., Shin C.M., Chang Y.S., Kim J.S., Jung H.C., Cho S.H. Inverse relationship between Helicobacter pylori infection and asthma among adults younger than 40 years: A cross-sectional study. Medicine (Baltim.) 2016;95:e2609. doi: 10.1097/MD.0000000000002609. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

