Mesenchymal stromal cells for the treatment of ocular autoimmune diseases
- PMID: 33775824
- PMCID: PMC8922475
- DOI: 10.1016/j.preteyeres.2021.100967
Mesenchymal stromal cells for the treatment of ocular autoimmune diseases
Abstract
Mesenchymal stromal cells, commonly referred to as MSCs, have emerged as a promising cell-based therapy for a range of autoimmune diseases thanks to several therapeutic advantages. Key among these are: 1) the ability to modulate innate and adaptive immune responses and to promote tissue regeneration, 2) the ease of their isolation from readily accessible tissues and expansion at scale in culture, 3) their low immunogenicity enabling use as an allogeneic "off-the-shelf" product, and 4) MSC therapy's safety and feasibility in humans, as demonstrated in more than one thousand clinical trials. Evidence from preclinical studies and early clinical trials indicate the therapeutic potential of MSCs and their derivatives for efficacy in ocular autoimmune diseases such as autoimmune uveoretinitis and Sjögren's syndrome-related dry eye disease. In this review, we provide an overview of the current understanding of the therapeutic mechanisms of MSCs, and summarize the results from preclinical and clinical studies that have used MSCs or their derivatives for the treatment of ocular autoimmune diseases. We also discuss the challenges to the successful clinical application of MSC therapy, and suggest strategies for overcoming them.
Keywords: Dry eye disease; Mesenchymal stem cell; Mesenchymal stromal cell; Ocular autoimmune disease; Sjögren's syndrome; Uveitis; Uveoretinitis.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures






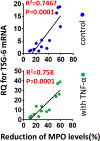



Similar articles
-
Advances in mesenchymal stem cell-derived extracellular vesicles therapy for Sjogren's syndrome-related dry eye disease.Exp Eye Res. 2023 Dec;237:109716. doi: 10.1016/j.exer.2023.109716. Epub 2023 Nov 10. Exp Eye Res. 2023. PMID: 37951337 Review.
-
Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases.Int J Mol Sci. 2023 Nov 7;24(22):16040. doi: 10.3390/ijms242216040. Int J Mol Sci. 2023. PMID: 38003230 Free PMC article. Review.
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles as novel cell-free therapy for treatment of autoimmune disorders.Exp Mol Pathol. 2021 Feb;118:104566. doi: 10.1016/j.yexmp.2020.104566. Epub 2020 Nov 6. Exp Mol Pathol. 2021. PMID: 33160961 Review.
-
Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases.Stem Cell Res Ther. 2022 Mar 7;13(1):101. doi: 10.1186/s13287-022-02782-7. Stem Cell Res Ther. 2022. PMID: 35255979 Free PMC article. Review.
-
Mesenchymal stem cells for treating autoimmune dacryoadenitis.Stem Cell Res Ther. 2017 Jun 5;8(1):126. doi: 10.1186/s13287-017-0593-3. Stem Cell Res Ther. 2017. PMID: 28583168 Free PMC article. Review.
Cited by
-
Single-cell transcriptomics unveil a unique molecular profile of mesenchymal stem/stromal cell-induced myeloid-derived immune suppressor cells.Mol Ther. 2024 Jun 5;32(6):1612-1613. doi: 10.1016/j.ymthe.2024.05.009. Epub 2024 May 24. Mol Ther. 2024. PMID: 38795702 No abstract available.
-
Effect of small extracellular vesicles derived from IL-10-overexpressing mesenchymal stem cells on experimental autoimmune uveitis.Stem Cell Res Ther. 2022 Mar 7;13(1):100. doi: 10.1186/s13287-022-02780-9. Stem Cell Res Ther. 2022. PMID: 35255957 Free PMC article.
-
Corneal Regeneration Using Adipose-Derived Mesenchymal Stem Cells.Cells. 2022 Aug 16;11(16):2549. doi: 10.3390/cells11162549. Cells. 2022. PMID: 36010626 Free PMC article. Review.
-
Enhanced immunosuppressive capability of mesenchymal stem cell-derived small extracellular vesicles with high expression of CD73 in experimental autoimmune uveitis.Stem Cell Res Ther. 2024 May 23;15(1):149. doi: 10.1186/s13287-024-03764-7. Stem Cell Res Ther. 2024. PMID: 38783393 Free PMC article.
-
Recent Advances of Adipose-Tissue-Derived Mesenchymal Stem Cell-Based Therapy for Retinal Diseases.J Clin Med. 2023 Nov 9;12(22):7015. doi: 10.3390/jcm12227015. J Clin Med. 2023. PMID: 38002628 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

