SMADS-Mediate Molecular Mechanisms in Sjögren's Syndrome
- PMID: 33801157
- PMCID: PMC8004153
- DOI: 10.3390/ijms22063203
SMADS-Mediate Molecular Mechanisms in Sjögren's Syndrome
Abstract
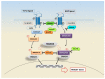
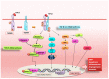
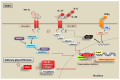
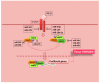
There is considerable interest in delineating the molecular mechanisms of action of transforming growth factor-β (TGF-β), considered as central player in a plethora of human conditions, including cancer, fibrosis and autoimmune disease. TGF-β elicits its biological effects through membrane bound serine/threonine kinase receptors which transmit their signals via downstream signalling molecules, SMADs, which regulate the transcription of _target genes in collaboration with various co-activators and co-repressors. Until now, therapeutic strategy for primary Sjögren's syndrome (pSS) has been focused on inflammation, but, recently, the involvement of TGF-β/SMADs signalling has been demonstrated in pSS salivary glands (SGs) as mediator of the epithelial-mesenchymal transition (EMT) activation. Although EMT seems to cause pSS SG fibrosis, TGF-β family members have ambiguous effects on the function of pSS SGs. Based on these premises, this review highlights recent advances in unravelling the molecular basis for the multi-faceted functions of TGF-β in pSS that are dictated by orchestrations of SMADs, and describe TGF-β/SMADs value as both disease markers and/or therapeutic _target for pSS.
Keywords: SMAD; Sjögren’s syndrome; TGF-β; epithelial-mesenchymal transition; fibrosis; inflammation.
Conflict of interest statement
The authors declare no conflict of interest
Figures
Similar articles
-
The TGF-β1 Signaling Pathway as an Attractive _target in the Fibrosis Pathogenesis of Sjögren's Syndrome.Mediators Inflamm. 2018 Nov 26;2018:1965935. doi: 10.1155/2018/1965935. eCollection 2018. Mediators Inflamm. 2018. PMID: 30598637 Free PMC article.
-
TGF-β Pathway in Salivary Gland Fibrosis.Int J Mol Sci. 2020 Nov 30;21(23):9138. doi: 10.3390/ijms21239138. Int J Mol Sci. 2020. PMID: 33266300 Free PMC article. Review.
-
IL-6 Contributes to the TGF-β1-Mediated Epithelial to Mesenchymal Transition in Human Salivary Gland Epithelial Cells.Arch Immunol Ther Exp (Warsz). 2020 Sep 10;68(5):27. doi: 10.1007/s00005-020-00591-5. Arch Immunol Ther Exp (Warsz). 2020. PMID: 32914376
-
Elevated CCL19/CCR7 Expression During the Disease Process of Primary Sjögren's Syndrome.Front Immunol. 2019 Apr 24;10:795. doi: 10.3389/fimmu.2019.00795. eCollection 2019. Front Immunol. 2019. PMID: 31068931 Free PMC article.
-
Immune and non-immune mediators in the fibrosis pathogenesis of salivary gland in Sjögren's syndrome.Front Immunol. 2024 Oct 14;15:1421436. doi: 10.3389/fimmu.2024.1421436. eCollection 2024. Front Immunol. 2024. PMID: 39469708 Free PMC article. Review.
Cited by
-
Extracellular matrix turnover in salivary gland disorders and regenerative therapies: Obstacles and opportunities.J Oral Biol Craniofac Res. 2023 Nov-Dec;13(6):693-703. doi: 10.1016/j.jobcr.2023.08.009. Epub 2023 Sep 12. J Oral Biol Craniofac Res. 2023. PMID: 37719063 Free PMC article.
-
Mechanisms of Yajieshaba in the treatment of liver fibrosis through the Keap1-Nrf2 signaling pathway.Front Pharmacol. 2023 May 9;14:1124015. doi: 10.3389/fphar.2023.1124015. eCollection 2023. Front Pharmacol. 2023. PMID: 37229248 Free PMC article.
-
E-Cadherin Signaling in Salivary Gland Development and Autoimmunity.J Clin Med. 2022 Apr 17;11(8):2241. doi: 10.3390/jcm11082241. J Clin Med. 2022. PMID: 35456333 Free PMC article. Review.
-
Duct ligation/de-ligation model: exploring mechanisms for salivary gland injury and regeneration.Front Cell Dev Biol. 2024 Jun 25;12:1399934. doi: 10.3389/fcell.2024.1399934. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38983787 Free PMC article. Review.
-
Analysis of MIR27A (rs11671784) Variant Association with Systemic Lupus Erythematous.Life (Basel). 2023 Mar 5;13(3):701. doi: 10.3390/life13030701. Life (Basel). 2023. PMID: 36983856 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

