Functional Coupling between DNA Replication and Sister Chromatid Cohesion Establishment
- PMID: 33802105
- PMCID: PMC8001024
- DOI: 10.3390/ijms22062810
Functional Coupling between DNA Replication and Sister Chromatid Cohesion Establishment
Abstract
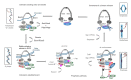
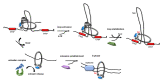
Several lines of evidence suggest the existence in the eukaryotic cells of a tight, yet largely unexplored, connection between DNA replication and sister chromatid cohesion. Tethering of newly duplicated chromatids is mediated by cohesin, an evolutionarily conserved hetero-tetrameric protein complex that has a ring-like structure and is believed to encircle DNA. Cohesin is loaded onto chromatin in telophase/G1 and converted into a cohesive state during the subsequent S phase, a process known as cohesion establishment. Many studies have revealed that down-regulation of a number of DNA replication factors gives rise to chromosomal cohesion defects, suggesting that they play critical roles in cohesion establishment. Conversely, loss of cohesin subunits (and/or regulators) has been found to alter DNA replication fork dynamics. A critical step of the cohesion establishment process consists in cohesin acetylation, a modification accomplished by dedicated acetyltransferases that operate at the replication forks. Defects in cohesion establishment give rise to chromosome mis-segregation and aneuploidy, phenotypes frequently observed in pre-cancerous and cancerous cells. Herein, we will review our present knowledge of the molecular mechanisms underlying the functional link between DNA replication and cohesion establishment, a phenomenon that is unique to the eukaryotic organisms.
Keywords: DNA replication; cell cycle; cohesin; replication proteins; sister chromatid cohesion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A matter of choice: the establishment of sister chromatid cohesion.EMBO Rep. 2009 Oct;10(10):1095-102. doi: 10.1038/embor.2009.207. Epub 2009 Sep 11. EMBO Rep. 2009. PMID: 19745840 Free PMC article.
-
Chl1 DNA helicase regulates Scc2 deposition specifically during DNA-replication in Saccharomyces cerevisiae.PLoS One. 2013 Sep 26;8(9):e75435. doi: 10.1371/journal.pone.0075435. eCollection 2013. PLoS One. 2013. PMID: 24086532 Free PMC article.
-
Establishment of sister chromatid cohesion at the S. cerevisiae replication fork.Mol Cell. 2006 Sep 15;23(6):787-99. doi: 10.1016/j.molcel.2006.08.018. Epub 2006 Sep 7. Mol Cell. 2006. PMID: 16962805
-
The Interplay of Cohesin and the Replisome at Processive and Stressed DNA Replication Forks.Cells. 2021 Dec 8;10(12):3455. doi: 10.3390/cells10123455. Cells. 2021. PMID: 34943967 Free PMC article. Review.
-
Fork it over: the cohesion establishment factor Ctf7p and DNA replication.J Cell Sci. 2007 Aug 1;120(Pt 15):2471-7. doi: 10.1242/jcs.011999. J Cell Sci. 2007. PMID: 17646671 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

