Neuropathophysiology of coronavirus disease 2019: neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection
- PMID: 33822324
- PMCID: PMC8021940
- DOI: 10.1007/s10787-021-00806-x
Neuropathophysiology of coronavirus disease 2019: neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection
Abstract
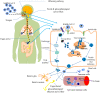
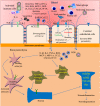
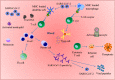
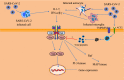
Coronavirus disease 2019 (COVID-19) is caused by the novel SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) first discovered in Wuhan, Hubei province, China in December 2019. SARS-CoV-2 has infected several millions of people, resulting in a huge socioeconomic cost and over 2.5 million deaths worldwide. Though the pathogenesis of COVID-19 is not fully understood, data have consistently shown that SARS-CoV-2 mainly affects the respiratory and gastrointestinal tracts. Nevertheless, accumulating evidence has implicated the central nervous system in the pathogenesis of SARS-CoV-2 infection. Unfortunately, however, the mechanisms of SARS-CoV-2 induced impairment of the central nervous system are not completely known. Here, we review the literature on possible neuropathogenic mechanisms of SARS-CoV-2 induced cerebral damage. The results suggest that downregulation of angiotensin converting enzyme 2 (ACE2) with increased activity of the transmembrane protease serine 2 (TMPRSS2) and cathepsin L in SARS-CoV-2 neuroinvasion may result in upregulation of proinflammatory mediators and reactive species that trigger neuroinflammatory response and blood brain barrier disruption. Furthermore, dysregulation of hormone and neurotransmitter signalling may constitute a fundamental mechanism involved in the neuropathogenic sequelae of SARS-CoV-2 infection. The viral RNA or antigenic peptides also activate or interact with molecular signalling pathways mediated by pattern recognition receptors (e.g., toll-like receptors), nuclear factor kappa B, Janus kinase/signal transducer and activator of transcription, complement cascades, and cell suicide molecules. Potential molecular _targets and therapeutics of SARS-CoV-2 induced neurologic damage are also discussed.
Keywords: Blood brain barrier disruption; COVID-19; Neuroinfection; Neuroinflammation; Neuropathogenesis; SARS-CoV-2.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Molecular Mechanisms of Proteins - _targets for SARS-CoV-2 (Review).Sovrem Tekhnologii Med. 2021;12(6):98-108. doi: 10.17691/stm2020.12.6.11. Epub 2020 Dec 28. Sovrem Tekhnologii Med. 2021. PMID: 34796023 Free PMC article. Review.
-
Neurological manifestations of SARS-CoV-2: complexity, mechanism and associated disorders.Eur J Med Res. 2023 Aug 30;28(1):307. doi: 10.1186/s40001-023-01293-2. Eur J Med Res. 2023. PMID: 37649125 Free PMC article. Review.
-
Tumor Necrosis Factor-Alpha Exacerbates Viral Entry in SARS-CoV2-Infected iPSC-Derived Cardiomyocytes.Int J Mol Sci. 2021 Sep 13;22(18):9869. doi: 10.3390/ijms22189869. Int J Mol Sci. 2021. PMID: 34576032 Free PMC article.
-
Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms.CNS Neurosci Ther. 2021 Jan;27(1):36-47. doi: 10.1111/cns.13569. Epub 2020 Dec 30. CNS Neurosci Ther. 2021. PMID: 33381913 Free PMC article. Review.
-
SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells.PLoS Pathog. 2020 Dec 7;16(12):e1009128. doi: 10.1371/journal.ppat.1009128. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33284859 Free PMC article.
Cited by
-
Hyperinflammatory Response in COVID-19: A Systematic Review.Viruses. 2023 Feb 16;15(2):553. doi: 10.3390/v15020553. Viruses. 2023. PMID: 36851766 Free PMC article. Review.
-
Quantum Dot Biomimetic for SARS-CoV-2 to Interrogate Blood-Brain Barrier Damage Relevant to NeuroCOVID Brain Inflammation.ACS Appl Nano Mater. 2023 Aug 7;6(16):15094-15107. doi: 10.1021/acsanm.3c02719. eCollection 2023 Aug 25. ACS Appl Nano Mater. 2023. PMID: 37649833 Free PMC article.
-
Ginkgo Biloba and Long COVID: In Vivo and In Vitro Models for the Evaluation of Nanotherapeutic Efficacy.Pharmaceutics. 2023 May 22;15(5):1562. doi: 10.3390/pharmaceutics15051562. Pharmaceutics. 2023. PMID: 37242804 Free PMC article. Review.
-
Sudden Hearing Loss Waves: The Effect of COVID-19 Infection and Vaccination on the Inner Ear.Adv Exp Med Biol. 2024;1457:265-283. doi: 10.1007/978-3-031-61939-7_15. Adv Exp Med Biol. 2024. PMID: 39283432 Review.
-
Potential of Endogenous Oxytocin in Endocrine Treatment and Prevention of COVID-19.Front Endocrinol (Lausanne). 2022 May 3;13:799521. doi: 10.3389/fendo.2022.799521. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35592777 Free PMC article. Review.
References
-
- Al Saiegh F, Ghosh R, Leibold A, Avery MB, Schmidt RF, Theofanis T, Mouchtouris N, Philipp L, Peiper SC, Wang Z-X, Rincon F, Tjoumakaris SI, Jabbour P, Rosenwasser RH. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323522. - DOI - PubMed
-
- Andries K, Pensaert MB. Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am J Vet Res. 1980;41:1372–1378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
